![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
8 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
What is a metallic bond? |
The attraction between metal ions and delocalised electrons in a metal |
|
|
|
Describe the structure of metals |
Metals consist of a giant structure. The atoms in a metal are arranged in a regular pattern. Metals are said to have giant structures because they have lots of atoms |

|
|
|
Bonding in metals |
In metals, the electrons in the outer shell of the atoms are delocalised. This means that they aren't associated with a particular atom or bond-they're free to move through the whole structure. There are strong forces of attraction between the positive metal ions and the negative electrons and these forces, known as metallic bonding, hold the metal structure together. |

|
|
|
Do metals have high melting and boiling points |
The electrostatic forces between the metal atoms and the delocalised sea of electrons are strong, so need lots of energy to be broken. This means that most compounds with metallic bonds have very high melting and boiling points, so they are generally solid at room temperature. |
|
|
|
Do metals conduct electricity? |
Metals have delocised electrons that are free to move through the whole structure. Because of this, thet are good conductors of thermal energy and electricity. The electrons carry the current or the thermal energy through the structure |
|
|
|
Are metals malleable? |
Metals consist of atoms held together in a regular structure. They form layers that are able to slide over each other. This means they are malleable-they can be bent and shaped, as well as hammered or rolled into shapes. |
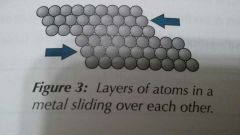
|
|
|
What is an alloy? |
A metal that is a mixture of two or more metals, or a mixture involving metals and non-metals |
|
|
|
Why are alloys harder than pure metals? |
Different elements have different size atoms. When another element is mixed with a pure metal, the new element atoms distort the layers of metal atoms, making it more difficult for them to slide over each other. |
|

