![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
49 Cards in this Set
- Front
- Back
|
Average molecular speeds
|
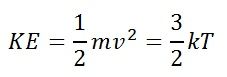
|
|
|
Gibbs Free Energy
|
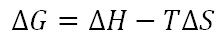
If G is negative --> RXN is spontaneous
If G is positive --> RXN is non-spontaneous If G is 0 --> Equilibrium (G=TS) |
|
|
Where do the distinct lines form an atomic emission spectra come from?
|
Upon falling back to ground state an electron emits a photon of a characteristic wavelength (lambda)
|
|
|
What phase is the absorption spectra used to identify elements in?
|
The gas phase
Blue Shift - Moving closer Red Shift - Moving Away |
|
|
Hund's Rule
|
orbitals filled with half spin first
|
|
|
Paramagnetic Vs Diamagnetic
|
Paramagnetic - Compounds with unpaired electrons have slight attraction to magnetic fields
Diamagnetic - Compounds with out unpaired electrons are slightly repelled from magnetic fields |
|
|
Formal Charge
|
Group in Periodic table - BARS - DOTS (covalent bonds - unpaired electrons)
|
|
|
Energy of a light quanta
|
E = hf or E = hv
h = plank's constant f = frequency |
|
|
Speed of light
|
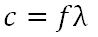
|
|
|
Electromagnetic energy of photons
|
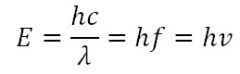
|
|
|
For every value of N (principle quantum number) there is _______ value (capacity) to hold electrons
|
2n^2
example: For n=1 2(1)^2 = 2 electrons |
|
|
Gas constant
|
R = 8.314 J/mol*K
R = 0.0821 L*Atm/mol*K |
|
|
Volume of a gas at STP
|
22.4 L
|
|
|
Total pressure is a sum of what
|
All the partial pressures
Pt = Pa + Pb + Pc + ...Pn |
|
|
Partial pressure
|
Pa = PtXa
Pt = Total pressure Xa = mol fraction (# mol A / # mol Tot) |
|
|
Pauli Exclusion Principle
|
No two electrons can have the same set of quantum numbers
|
|
|
Aufbau Principle
|
Governs how orbitals are filled.
|
|
|
Electron Affinity
|
Energy released when an electron is added to the outer shell of an atom
Increases Left to Right across periodic table |
|
|
Reaction Quotient "Q"
|
Q = [product] / [reactant]
|
|
|
Normality equation
|
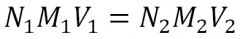
Normality is # equivalents/L
You get normality by nultiplying the (# equivalents/mol * molarity) Remember M1V1 = M2V2 |
|
|
Energy of C-H bonds
|
The more S character a bond has the lower in energy it is.
ALKYNE < ALKENE < ALKANE ALKYNE - 50% S ALKENE - 33.3% S ALKANE - 25% S |
|
|
Arrhenius Acid/Base
|
Acid - produces H
Base - produces -OH All about water |
|
|
Bronsted-Lowry Acid/Base
|
Acid - Donates H
Base - Accepts H Works for organic |
|
|
what is an amphoteric species
|
One that can act as an acid or a base
|
|
|
pH
|
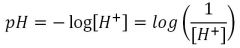
|
|
|
Logarithm math (with pH)
|
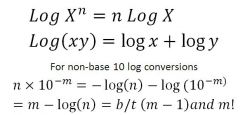
|
|
|
Small Ka and Kb values indicated a strong or weak acid or base?
|
Small Ka = weak acid
Small Kb = weak base K is [products]/[reactants] |
|
|
Ka (conj. acid) X Kb (conj. base) = ????
|
= Kw = 10^(-14)
|
|
|
equivalent weight
|
the gram equivalent weight is the mass of a compound that produces one equivalent (mole of charge)
ex: H2SO4 is divalent, molar mass = 98g/mol. Since it's divalent the equivalent weight is 98/2 = 49g. Thus 49 g will give one mole of charge (H+) |
|
|
Titration equation
|
NaVa = NbVb
N = normality V = volume |
|
|
In titration when does the indicator change color?
|
At the end point, NOT the equivalent point
|
|
|
TITRATION (titrand & titrant)
Strong Acid and Strong base |
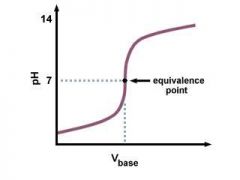
Equivalence point = pH 7
|
|
|
TITRATION (titrand & titrant)
Weak Acid & Strong Base |
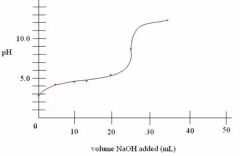
Equivalce point = pH > 7
|
|
|
TITRATION (titrand & titrant)
Weak base & Strong Acid |
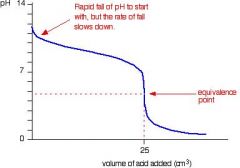
equivalence point = pH < 7
|
|
|
TITRATION (titrand & titrant)
Polyprotic Base & Strong Acid |
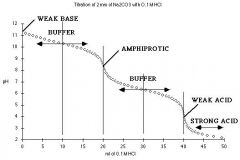
The tirtation of acidic or basic amino acids will look similar but with 3 equivalence points.
|
|
|
Buffer
|
Either a mixture of:
1) A weak acid and its salt ex: acetic acid & Sodium Acetate 2) A weak base and its salt ex: ammonioa & ammonium chloride |
|
|
Henderson-Hasselbalch Equation
|
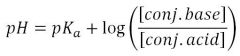
Used to estimate the pH or pOH of a solution in the buffer region where concentrations are approx. equal
The one above is for a weak acid buffer solution. Reverse it for a weak base. |
|
|
Arrhenius Acid/Base
|
Acid: H donor
Base: OH donor |
|
|
Bronsted-Lowry Acid/Base
|
Acid: H Donor
Base: H Acceptor |
|
|
Kw
|
Equilibrium constant of autoionization of water
NOTE all "K" are ratio of [products]/[reactants] Kw = Ka x Kb all K are temp dependent |
|
|
Normality
|
Equivalents per Liter
Norm = Molarity x eq/mol |
|
|
Cathode is always the site of
|
Reduction!!
RED CAT AN OX |
|
|
Emf (E)
|
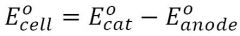
If E > 0 then spontaneous (opposite of Gibbs)
|
|
|
Gibbs Energy (cell)
|
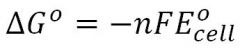
|
|
|
Gibbs Cell related to Keq
|
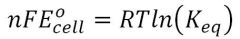
|
|
|
Total Charge transferred in electrolysis
|
I x t = n x F
I = current t = time n = number of moles of electrons F = Faraday ~ 100,000 |
|
|
Ortho Para (activating) directing (pic)
|
|
|
|
Ortho & Para activating directors
[pic] |
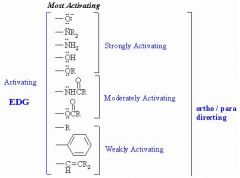
|
|
|
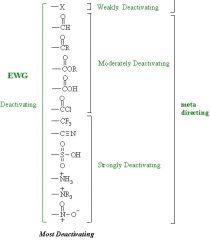
Meta de-activating directors
[pic] |
|

