![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
10 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
What is Beta-galactosidase and what does it do? |
An enzyme that cleaves the milk disaccharide lactose into glucose and galactose. These sugars can then be metabolized for energy and ATP production. |
|
|
|
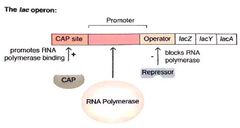
How is the lac operon repressed? How can the lac operon be derepressed? Is the lac operon fully active at this point? If not, when does it become fully active? |
The lac1 regulatory protein binds to the operator downstream of the promoter blocking transcription. When lactose is present, it's byproduct allolactose allosterically binds to the repressor causing it release from lac operon. The operon is not fully active until cAMP binding protein (CAP) binds to a site upsteam of the promoter facitating RNA binding. This only happens when ATP levels are low. |
|
|
|
What is isopropyl thiogalactosidase (IPTG)? What does it do? |
IPTG is an analog of lactose and can interactswith lac1 preventing it from bindig to the operator. |
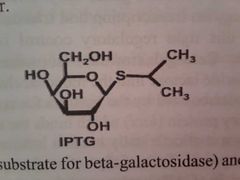
This looks like the sugar... |
|
|
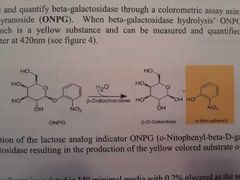
How can we observe and quantify beta galactosidase activity? What chemical did we use? What happens when this chemical comes into contact with Beta-gal? |
Through a colourmeric assay using o-Nitrolphenyl-beta-D-galactopyranoside (ONPG). When Beta-gal hydrolysis' ONPG it produces o-Nitrophenol which is yellow so we can measure it using a spectrometer at 420nm. |
|
|
|
What was the E.coli growing in before we added anything? What was its O.D.? |
The e.coli was grown in M9 minimal media with glycerol as the only carbon source and grown to an optical density of 0.15 at 600nm O.D. |
|
|
|
Why do we add chloroform and SDS to the sample after it has been mixed? |
To lyse cells and release cytoplasmic contents into solution. |
Look at #6 in Week 1 procedure |
|
|
What temperature do we store the treated cultures once they have been lysed? |
At -20°C |
|
|
|
Describe the Beta-gal assay procedure. |
Thaw cell extracts in 30°C water bath. Obtain 10 tt's with Z buffer and label. Transfer 0.5ml of cell extract to appropriate Z buffer assay tube. Add ONPG to 0min sample. At 1min intervals add OPNG to all samples and record start time. Incubate samples until a yellow colour appeared then stop the reaction by adding 2ml stop buffer. Record time. |
|
|
|
What was in the blank solution? |
A solution of Z buffer, ONPG and stop buffer was used to zero the spectrometer. |
|
|
|
What is the formula for specific activity? |
Specific activity = A420 / min x sample volume (ml) x OD600 |
|

