![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
36 Cards in this Set
- Front
- Back
|
In what state do molecules exhibit highly ordered packing? |
Solid |
|
|
In what states don't molecules have packing order? |
Gas and Liquid |
|
|
What are the physicochemical properties impacted by solid state properties? |
-Solubility -Dissolution rate -Bioavailability -Stability -Melting Point -Surface Activity -Density -Electrostatic, mechanical and optical properties -Formulaiton Design |
|
|
What are the two structures that a solid can be? |
Amorphous or Crystalline |
|
|
What can crystalline structures exist as? |
-Polymorphs -Co-crystals -Solvates, Hydrates |
|
|
What are the features of the crystalline state? |
-Molecules arranged in orderly defined manner, with same repeating units -Lower potential energy than amorphous state |
|
|
What is polymorphism? |
Repeating units can be arranged differently within the crystalline form of the same substance |
|
|
What do different crystalline forms of the same substance have? |
Different melting points-> the temperature at which the lattice is broken down |
|
|
What are the three methods of crystallisation? |
-Supersaturated solution -Crystallisation through cooling molten sample below its melting point -Precipitation of solids in solutions |
|
|
What medicinal forms are crystallised by cooling the molten sample below its melting point? |
-Suppositories -Creams -Gels |
|
|
How are solids in solution precipitated during crystallisation? |
-Evaporation of liquid -Addition of anti-solvent -Solubility of sample changed by temp/pressure/pH of the system |
|
|
What are the two main steps of the crystallisation process? |
-Nucleation -Growth |
|
|
What is Nucleation? |
-First step in forming crystals -A small mass clusters together to form a 'nuclei' on which a crystal can grow THE SOLUTION MUST BE SUPERSATURATED |
|
|
What can crystalline polymorphs possess? |
-Different crystalline forms -Different Packing Pattern -Different lattice energies -Different properties |
|
|
What are the features of a stable form crystalline polymorph? |
-High melting point -Slower dissolution rate |
|
|
What are the features of a metastable form crystalline polymorph? |
-Lower melting point -Faster dissolution -Increase in apparent solubility |
|
|
What factors determine how fast the metastable form will turn into the stable form? |
-Energy difference between the two -Environmental conditions |
|
|
What does Monotropic Polymorphism mean? |
Only one stable polymorphoc form (metastable will convert into stable over time) |
|
|
What does Enantropic Polymorphism mean? |
Material reversibly transformed between alternative stable forms. Less common. |
|
|
What is a hydrate? |
Where the solvent is water |
|
|
What does monohydrate mean? |
1 molecule water: 1 molecule substance |
|
|
What does dihydrate mean? |
2 molecules water: 1 molecules substance |
|
|
What is a solvate |
Where another solvent instead of water is used (eg: organic solvent like ethanol) |
|
|
What does pseudopolymorphism mean? |
The difference between hydrates and anhydrous forms |
|
|
What are the features of the Amorphous State? |
-Lower packing efficiency -Greater intermolecular distance -Greater molecular mobility -Greater potential energy -Often higher solubility |
|
|
What causes solids to become amorphous? |
-Insufficient solidification time for molecules to form order -Lack of kinetic energy to overcome barrier between crystal-liquid interface -The crystalline process has been broken through processing |
|
|
What type of materials are commonly amorphous? |
Those with a low molecular weight |
|
|
What type of compounds exhibit semi-crystalline structures? |
Larger molecular weight compounds, such as polymers |
|
|
What do semi-crystalline structures have? |
Both an ordered and disordered region |
|
|
What is the Glass Transition Temperature? |
The characteristic temperature at which amorphous forms exhibit a major change in properties |
|
|
What does a temperature lower than the glass transition temperature lead to? |
Glassy brittle state Lower mobility of molecules (slower conversion to crystaline form) |
|
|
What does a temperature higher than the glass transition temperature lead to? |
Rubbery High mobility of molecules |
|
|
What is a plasticiser? |
A substance added to reduce the glass transition temperature of an amorphous material (Water is a good plasticiser) |
|
|
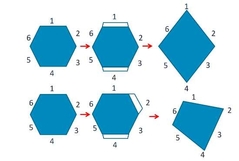
What is crystal habit? |
The eternal shape of the crystal -Due to rate of crystal growth at different faces -Influenced by crystalline conditions -Can be different for different internal packing -Can also be different for the same packing |
|
|
What does Crystal Habit affect? |
Drug Properties: -Dissolution rate -Powder flow -Stability |
|
|
Changes in crystal habit |
|

