![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
128 Cards in this Set
- Front
- Back
|
At what level of elevated serum bilirubin do you see scleral icterus, and is it conjugated or unconjugated?
|
2mg/dL conjugated bilirubin
|
|
|
When do you notice mucus membrance and cutaneous jaundice?
|
2-4mg/L (40-80 umol/L)
|
|
|
Describe the 4 sources of bilirubin and what % each source accounts for?
|
Bilirubin comes from the metabolism of…. Hb of senescent erythrocytes (80%)
Breakdown of haemoproteins in the liver (20%) Ineffective erythropoiesis (2—3%) Haemoproteins in the extrahepatic tissue such as myoglobin (<1%) |
|
|
What is cholestasis and how does it present?
|
Cholestasis is the Impairment of bile formation or bile flow
May present with fatigue, pruritis or jaundice…or picked up on routine blood screening |
|
|
What sort of hyperbilirubiniaemia is seen in cholestasis and why?
|
Conjugated hyperbilirubinaemia
Excretion of conjugated bilirubin is the rate-limiting step of bilirubin clearance. During cholestasis, conjugation of bilirubin continues but excretion is reduced. The mechanism by which conjugated bilirubin regurgitates into serum is unclear, but it may differ according to the disease etiology. |
|
|
Describe hepatocyte conjugation of bilirubin?
|
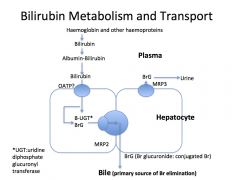
Hepatocyte take up Br and conjugate to glucuronide and excrete Br diglucuronide in bile into the duodenum (MRP3)
|
|
|
What happens to the conjugated bilirubin once it is excreted into the duodenum?
|
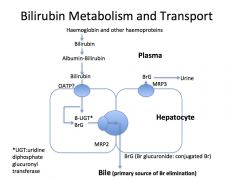
In terminal ileum and colon, bacteria break down Br to urobilinogen, 80% excreted in faeces. Remaining 20% reabsorbed and excreted in bile and urine (enterohepatic circulation of urobilinogen)
|
|
|
What is Gilberts Syndrome?
|
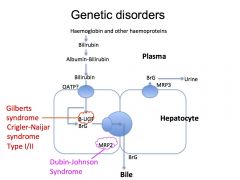
A benign disorder which presents as unconjugated hyperbilirubinaemia (mainly in times of stress) due to reduction in transcription of the UGT:uridine diphosphate glucuronyl transferase enzyme
|
|
|
What is Crigler Naijar Syndrome?
|
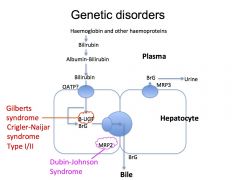
Crigler Naijar syndrome is a paediatric inherited form of non-hemolytic jaundice, which results in high levels of unconjugated bilirubin and often leads to brain damage in infants.
It is due to complete deficiency of UGT:uridine diphosphate glucuronyl transferase. |
|
|
What is Dubin- Johnson Syndrome?
|
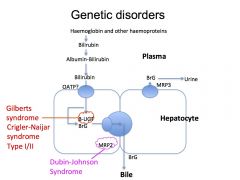
It is a syndrome that causes conjugated hyperbilirubinaemia as there is a defective transport protein MRP2 which prevents the excretion of the bilirubin into the bile.
|
|
|
What is heptatocellular cholestasis?
|
impairment of bile formation
|
|
|
What LFTS suggest cholestasis?
|
ALP>1.5 X normal
GGT > 3 X normal Note – if GGT is elevated in isolation usually due to alcohol or drugs – hence need no further investigation |
|
|
What are the 8 commonly recognized causes of hepatocellular cholestasis?
|
Sepsis, Viral hepatitis, Alcoholic or non-alcoholic steatohepatitis, Drug- or parenteral nutrition-induced cholestasis, Genetic disorders: e.g. Wilson’s disease, BRIC, PFIC, ABCB4 deficiency, Pregnancy, Cirrhosis and Benign infiltrating disorders: amyloidosis, sarcoidosis and other granulomatoses, storage diseases
|
|
|
What is cholangiocellular cholestatsis and name important causes?
|
Impairment of bile flow due to dysfunctional components of the bile ducts i.e. cells
Primary sclerosising cholangitis Primary biliary cirrhosis (antibodies) Cystic fibrosis Infection, Stones, Ischaemia – all causing secondary cholangitis |
|
|
What are some of the causes of intrahepatic cholestasis in infacy and childhood?
|
Metabolic disorders – i.e. alpha 1 antitrypsin deficiency, storage diseases, cystic fibrosis
Paucity of bile ducts – Alagille syndrome infections Toxic – parental nutrition or drugs idiopathic neonatal hepatitis |
|
|
What are the LFTs that suggest Acute Drug induced cholestatic injury?
|
Serum AP (alkaline phosphatase) > 2
OR ALT/AP rations < 2 |
|
|
what determine your suseptinility to drug induced cholestatic injury?
|
both environmental and genetic factors
|
|
|
Which 2 drugs are known to cause acute intrahepatic cholestatsis without associated hepatitis?
|
oestrogen and anabolic steroids
|
|
|
Which 6 drugs are known to cause acute intrahepatic cholestatsis with associated hepatitis?
|
isoniazid, tricyclic antidepressents, cabamezapine, metformin,augmentin, atorvastatin
|
|
|
Which 2 drugs are known to cause acute intrahepatic cholestatsis with bile duct injury?
|
flucloxacillin and pioglitazone
|
|
|
Describe the jaundice associated with pregnancy?
|
It can be due to….
1 - intrahepatic cholestasis – seen in the 3rd trimester presenting with pruritis and occasional jaundice. 2 – Acute fatty liver of pregnancy – also seen in the 3rd trimester – fatal unless pregnancy delivered 3 – 10% of preclamspia has liver involvedment also required prompt delivery 4 - Hyperemesis gravidarum (HG) - a severe form of morning sickness which can occasionally cause jaundice |
|
|
What are Choledocholithiasis?
|
Choledocholithiasis is the presence of at least one gallstone in the common bile duct. The stone may be made up of bile pigments or calcium and cholesterol salts.
|
|
|
What are the 2 most common causes of obstructive jaundice and how are they detected?
|
Gallstones – dilatation of the bileducts on US
Cancer of the head of the pancreas |
|
|
True or False? at the age of 75% there is 90% loss of sinoatrial node cells
|
TRUE
|
|
|
When is the best time to operate following emergency admission of an elderly patients?
|
48 hours after admission – note – not immediately
|
|
|
Fever swings over 14 days post operative suggests what?
|
abscess formation
|
|
|
What are the 2 main types of cells in the gastric glands stomach, where are they located and what do they secrete?
|
Parietal Cells (oxyitic cells) – located in the middle 1/3 glands and secrete H+ and intrinsic factor
Chief Cells – located in the bottom 1/3 of the glands and secrete pepsinogen |
|
|
Which cells secrete HCO3- in the stomach?
|
the surface epithelium
|
|
|
How does the surface epithelia secrete HCO30- in the stomach – and what is it similar too?
|
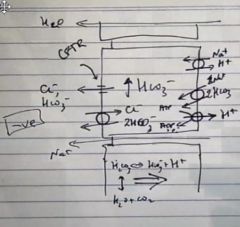
HCO3- is pumped into the cell with Na+ using the Na+ gradient
H+ is pumped out of the cell with Na+ and also with a H+ ATPase, causing there to be a increase in HCO3- in the cells The HCO3- is the secreted out of the cell via a CL- and HCO3- channel (CFTR) and also a CL- and HCO3- exchanged (hence Cl- is used to cycle the HCO3- out of the cells) This mechanism is similar to bile ducts and pancreatic ducts |
|
|
What ion is associated with brining HCO3 – into gastric surface epithelial cells, and which ion is responsible for its secretion out of these cells and what regulated the whole system?
|
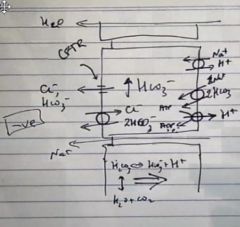
Na+ in
Cl- out cAMP regulates the whole system and acts via PKA |
|
|
Secretion of HCO3- by gastric surface epithelial, bile ducts and pancreatic ducts is regulated by cAMP (activating PKA)– what stimulates cAMP in these systems?
|
Gastric – PGE2
Bile and Pancreatic ducts – Secretin |
|
|
Describe a resting parietal cell and a activated parietal cells (i.e. via histamine, gastrin or Ach)?
|
ACTIVATE w5g
|
|
|
What activates parietal cells?
|
Histamine, Gastrin and Ach
|
|
|
What do Parietal cells normally secrete when not activated?
|
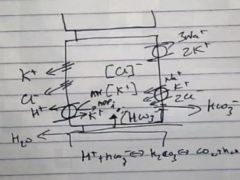
K+ and Cl-
|
|
|
Describe how activated parietal cells secrete H+?
|
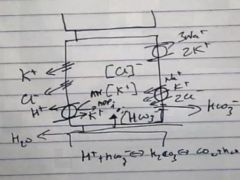
It uses the K+ that it has secreted to pump K+ into the cells exchanging it for a H+ with the K+ H+ ATPase
|
|
|
Why is there a basolateral HCO3- channel in parietal cells?
|
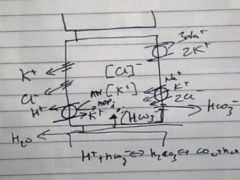
To maintain the equilibrium in the cell, balancing the H+ secretion
|
|
|
What drives H+ secretion by parietal cells? Is it the same as what drives HCO3 secretion by the surface epithelium?
|
H+ secretion by the parietal cells is controlled by intracellular calcium levels
Whereas cAMP controls HCO3 secertion by the surface epithelium |
|
|
Where is the intrinsic factor B12 complex taken up?
|
The terminal ileum
|
|
|
What are the 2 important cells tyrps of the small intestine and what are their major junctions?
|
Villous cells – absorb fluid electrolytes and nutrients i.e. glucose
Crypt cells – Secretory and stem cells for the intestinal epithelium (villous cells) |
|
|
Why does it take 3-7 days to recover from rotavirus?
|
Because rotavirus destroys the villous cells and it takes 3-7 days for the crypt cells to differentiate to replace the destroyed cells
|
|
|
What is the pathogenesis of cholera?
|
Irreversibly activates the secretory crypt cells leading to increased fluid secretion and inhibition of differentiation
|
|
|
Which two apical proteins are responsible for fluid and electrolyte absorption by the villous cells of the small intestines and whats an important point to know about this?
|
A Cl- HCO3- exchanger and a H+ Na+ exchanger - Note there is no voltage generation here – the small intestine tends to be low voltage
|
|
|
By which cells and how is glucose absorbed in the small intestines?
|
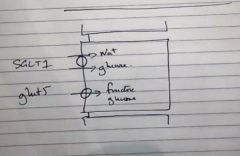
By the villous cells via the SGLT1 trasporter (cotransports Na+ ang glucose) and GLUT5 (channel for glucose and fructose)
|
|
|
Is fructose absorption active or passive in the small intestinal villous cells?
|
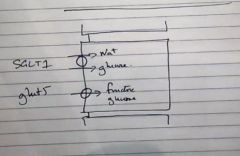
Passive through GLUT5 and GLUT2
|
|
|
How are tripeptides absorbed in the small intestine?
|
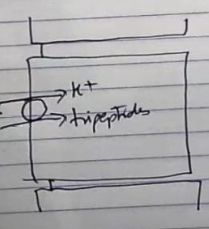
via a proton gradient
|
|
|
Explain the lactose intolerance associated with a rotavirus infection?
|
When rotavirus destroys the villus cells, it destroys the brush boarder when lactase is located (lactase is rewuired for lactose digestion and absorption), also following regeneration of the villous cells via crypt cell differentiation the brush boarder is the last to regenerate hence the lactose intolerance remains the longest
|
|
|
What % of gall stones are seen of Plain AXR?
|
20%
|
|
|
If only 20% of gall stones are seen on plain AXR why is it used?
|
To exclude other pathology i.e. bowel obstruction
|
|
|
How do you screen those with Cirrhosis for HCC and if it is positive what is the next test?
|
Ultrasound and Alpha-feto protine
The next step is Multi ring CT or MRI |
|
|
Describe the sensitivities of CT MRI and PET for Liver Metasitisis?
|
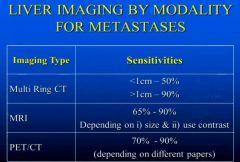
|
|
|
What is the most common for of benign lesion of the liver and how is it identified?
|
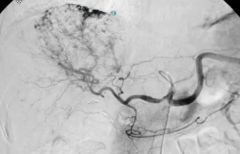
Haemangioma
On CT is enhances with contrast from the outside in On T2 MRI very bright |
|
|
How do you identify a liver nodule on imaging?
|
high signal intensity in the liver on T1 MRI
|
|
|
What does HCC look like on imaging?
|
Moderate to high signal on T2 MRI
|
|
|
Which is the outermost layer of muscle of the GI Tracts?
|
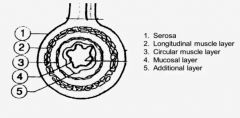
The longitudinal muscle layer
|
|
|
Describe the innervation and control of motility along the GIT starting with a bolus of food entering the tract?
|
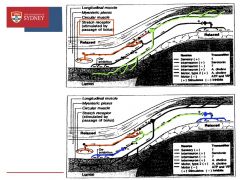
Stretch receptors in myenteric plexus (between the circular and longitudinal muscle layers) sense the passage of a bolus and stimulate neurones to release an unknown NT which stimulate serotonergic interneurones which then stimulate motor neurones to release ACh and cause circular muscle contraction
Stretch receptors also cause activation of Cholinergic interneurones which in turn stimulate inhibitory neurones to cause relaxation of circular muscle in front of the bolus and also behind the stretch receptors |
|
|
What are the 4 receptors in the Chemoreceptor Trigger Zone which is not protected by the BBB?
|
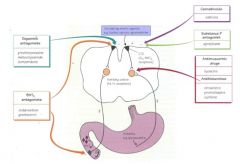
D2, 5HT3, Cannabanoid and Substance P
|
|
|
What drugs act on which receptors in the Vomiting center located in the medulla protected by the bbb?
|
Antimuscarinic (M) and anti histamines (H1)
|
|
|
What is Metaclopramine?
|
Dopamine receptor antagonist in the CNS which acts on the Chemoreceptor Trigger Zone
Also – stimulates motility in upper GI tract – increases gastric emptying rate and reducing intestinal transit time – causes ACh release in the myenteric plexus |
|
|
What are the side effects of Metaclopramide?
|
restlessness, drowsiness, dizziness, head ache. Anxiety and agitation can occur if given IV
Extrapyramidal effects – dystonic reaction, tardive dyskinesia. Parkinson-like symptoms can occur with prolonged use (rigidity, tremor) and can take several months to resolve after withdrawal |
|
|
What is Domperidone?
|
Dopamine receptor antagonist in the chemoreceptor trigger zone, and a direct stimulant of gastric motility
It is used as an alternative to metaclopramide, not quite as effective but used because it has Less side effects because it cannot cross the blood brain barrier to generate extrapyramidal side effects |
|
|
Name 5, 5HT3 Receptor antagonists?
|
Ondansetron, Dolansetron, Granisetron, Tropisetron and Palonosetron.
|
|
|
What is Ondansetron?
|
a 5HT3 receptor antagonist that acts on the chemoreceptor trigger zone to reduce the vomiting reflex
|
|
|
What are the adverse effects of ondasertron?
|
headache and constipation, flushing and visual disturbances may occur with IV injection
|
|
|
What is Aprepitant?
|
A Substance P Antagonist
|
|
|
What is Hyoscine and what is it used for?
|
mAChR antagonist (Antimuscarinic) used to trear motion sickness
|
|
|
What is Cinnarisizine?
|
An antihistamine used for motion sickness
|
|
|
What is Imodium?
|
Loperamide – an opiod like drug which exploits the constipation side effects assoisated with opiods
|
|
|
What is the mechanism of action of Imodium?
|
Decreases the tone of longitudinal muscles, increases the tone of circular muscles, prolonged transit and increases water absorbed
It also crosses the blood brain barrier, but is actively pumped back out by P-glycoprotein |
|
|
What is Lomotil?
|
A mixture of Atropine and Dipheboxylate – a mAChR antagonist with a mu receptor agonist
|
|
|
Name 5 osmotic laxatives?
|
Magnesium sulphate, macrogel 3350, PEG, lactulose, sorbitol
|
|
|
Describe how lactulose works?
|
Lactulose is metabolised to lactic acid and sugars by colonic bacteria - lactic acid reduces colonic pH and reduces absorption of ammonia and toxic amines – used for the treatment of hepatic encephalopathy
|
|
|
What are these drugs bisacoydl, sodium picosulfate, anthraquinone derivatives (eg. senna) and how do they work?
|
They are stimulant laxatives…
Unknown action – but stimulate enteric nervous system and increase motility – effect is rapid and local – none are absorbed to any significant extent |
|
|
What sort of contractions occur in the gastric resoivour of the stomach and then antral region?
|
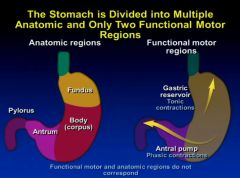
|
|
|
Which drug can also cause yellow skin (without icterus)
|
Quinacrine
|
|
|
Which drug can also cause yellow skin (without icterus)
|
Quinacrine
|
|
|
Describe IgA nephropathy and is it a nephrotic or nephritic condition?
|
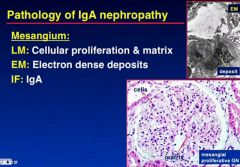
|
|
|
What infection is this and what are the arrows pointing to?
|
Strongdiloides infection (worm cut)
Eosinophils |
|
|
What are the biological detergents and what are they made from?
|
Bile salts are the biological detergents from cholesterol.
|
|
|
What is the specific transporter for bile acid secretion and cholesterol secretion by the hepatocytes?
|
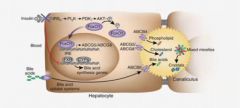
Bile Acids - ABCB1
Cholesterol - ABCG5/8 |
|
|
What % of bile salts are lost in the faeces and what happens to the rest?
|

Only 5% of bile salts are lost in the faeces
The remaining 95% is taken back up into the enterohepatic circulation |
|
|
In Liver failure why does bile salt levels rise to greater than 10 umol/L (which leads to puritis)?
|
Because there is defective bile acid secretion and or defective reuptake form the enterohepatic circulation
|
|
|
Describe how bilirubin is formed?
|
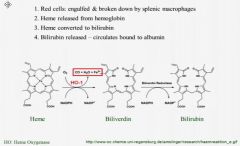
|
|
|
What is bilirubin conjugated to in hepatocytes?
|
Glucuronic acid
|
|
|
In what 5 liver diseases is Insulin resistance and type 2 diabetes seen in?
|
Non-alcoholic fatty liver diease
Non-alcoholic steatotic hepatitis Hepatitis C Hemochromatosis Cirrhosis |
|
|
In what % of cirrhosis is there insulin resistance and subsequent type 2 diabetes?
|
95% of cirrhosis has insulin resistance
30% have type 2 diabetes |
|
|
IS there an associated risk between type 2 diabetes and HCC and what affects this risk?
|
YES – 2X increase in risk of developing HCC in type 2 diabetes
Metformin use though reduces this risk substantially |
|
|
What are the 7 most important proteins synthesized by the liver (be specific for 2)?
|

|
|
|
While there is hyperglycaemia in liver failure due to insulin resistance, what happens during end stage liver failure?
|
hypoglycaemia
|
|
|
How long is the transit time of food in the stomach vs small bowel vs colon?
|

|
|
|
Describe the pH in the stomach vs small bowel vs colon?
|

|
|
|
Describe the flora found in the stomach vs small bowel vs colon?
|

|
|
|
Food Poisoning presents with rapid onset vomiting and dirrhoea but what is an important negative finding?
|
No fever
|
|
|
What are the 4 most common causes of food poisoning and what foods do they usually come from?
|
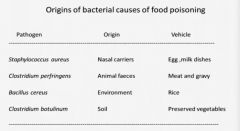
|
|
|
What can the Amanita Phylloides Mushroom cause that leads to death?
|
hepatotoxicity
|
|
|
Blood group O is at greater risk of which infections?
|
cholera, entero-hamorrhagic e. coli or norovirus
|
|
|
what is the most common cause of viral enteritis in infants?
|
Rotavirus
|
|
|
what is the most common cause of viral enteritis at any age?
|
Norovirus
|
|
|
What is the most common type of e.coli enteritis in the developed world?
|
EHEC – Enterhaemorrhagic ecoli
|
|
|
What type of ecoli infection is seen in AIDS patients and infants under 6 months?
|
Enteroaggregative E coli – EaggEC
|
|
|
What type of ecoli infection causes watery diarrhea in in fants under 1 year?
|
APEC – Enterpathogenic Ecoli
|
|
|
What are the two most common types of toxigenic diarrhea and where do they effect and hence what is the type of diarrhoea?
|
V. Cholerae and E.coli
They effect the proximal small bowel mostly – therefore profuse watery diarrhea |
|
|
What are the 3 common causes of invasive diarrhea and where do they effect and hence what is the type of diarrhoea?
|
Campylobacter, Salmonella and Yersinia
Effect the terminal small bowel and the proximal colon- hence there is moderate stoll volume and there is colickly abdominal pain |
|
|
What are the two most common causes of protozoan enteritis?
|
Cryptosporidium and giardia
|
|
|
When is salmonella most common?
|
In the summer months
|
|
|
A Child presents with slowed growth and escezma – what is a suggested cause?
|
Cows milk protein intolerance
|
|
|
What do you see in post streptococcal glomerulonephritis?
|
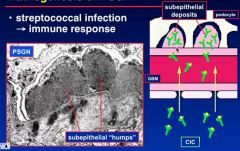
subepithelial “humps” of immune complexes
|
|
|
Describe the renal pathology in PSNG - post streptococcal glomerulonephritis on LM microscopy and Immunoflourence?
|
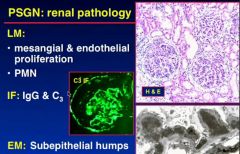
|
|
|
Describe the presentation and diagnositic test results?
|
Acute nephritis – haematuria and proteinuria
Presence of infection – ASOT and Anti DNAse B tires C3 levels |
|
|
What are the two pathological aspects of Good pastures disease?
|
Focal Necrotising glomerulonephritis and pulmonary hemorrhage
|
|
|
What is the cause of good pastures syndrome?
|
antibodies against the glomerular basement membrane protein - type IV collage
|
|
|
How do you treat goodpastures syndrome?
|
Clear antibodies by plasma exchange
|
|
|
How does Goodpastures syndrome look different to other antibody mediated glomerulonephritis on Immunofluorences?
|
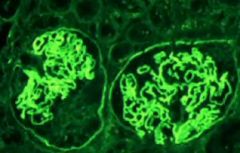
there is linear IgG located – not bumping like in Post streptococcal
|
|
|
What pathology occurs in goodpastures syndrome?
|
focal glomerular necrosis and crescent formation
|
|
|
What is associated with goodpastures syndrome that can lead to death?
|
Pulmonary hemorrhage
|
|
|
What type of GN accounts for 25% of all GN and has a white and Asian prevalence?
|
IgA nephropathy
|
|
|
Is IgA nephropathy more common in males or females?
|
Males – 3:1
|
|
|
What age group is commonly effected with IgA nephropathy?
|
20-30 year olds
|
|
|
What is the cause of IgA nephroaphy?
|
idiopathic
|
|
|
What is a synpharyngitic presentation and what GN is it associated with?
|
IgA nephropathy
It is a presentation of recurrent macroscopic hematuria, with a viral LRTI or Gastroenteritis |
|
|
What is Henoch Schonlein Purpura?
|
Henoch-Schonlein purpura is a childhood disease that involves purple spots on the skin (vasulitic rash), joint pain, gastrointestinal problems (colic) , and glomerulonephritis – IgA Nephropathy
|
|
|
What accounts for 20-40% of all hematuria and whats its prognosis?
|
Thin membrane glomerulopathy – very good prognosis
|
|
|
What is crescent cell formation caused by?
|
Parietal epithelial and Mononuclear cell proliferation
|
|
|
What are the 3 ANCA related small vessel vasculitis’ that can effect the kidneys?
|
Wegeners granulomatosis, microscopic polyangiitis and Churg-Strauss
|
|
|
What are the 3 Immune complex small vessel vasculitis’ that can effect the kidneys?
|
Lupus (SLE), Cyroglobulinaemia and Henoch Scholein Purpura
|
|
|
How do you tell the difference between IgA nephropathy and SLE nephropathy?
|
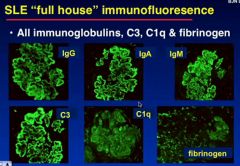
in IgA nephropathy only IgA is ideinfitable on IF
Whereas in SLE there is a “full house” of all immunoglobulins, C3, C1q and Fibrinogen in the IF of the capsule |
|
|
What pathological findings point to membranous GN?
|
subendothelial deposits causing spikes of the GBM
|
|
|
What are the 3 “absolute” definitions of failure to thrive
|

|
|
|
What pathology do you seen on LM, EM and IF in IgA nephropathy
|
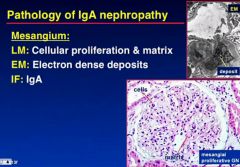
|

