![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
100 Cards in this Set
- Front
- Back
|
What 3 things do the islets of Langerhan secrete?
|
insulin, glucagon and somatostatin.
|
|
|
The islets of Langerhans make up approximately what % of the total weight of the pancreas?
|
2% - They are scattered throughout the exocrine pancreas.
|
|
|
What do the acini secrete?
|
The acini secrete an isotonic NaCl rich fluid and a wide range of proteins.
|
|
|
Describe the mechanism of secretion of the acini?
|
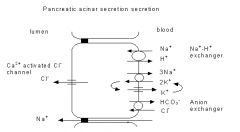
The secretion by the acini relies upon the uptake of Cl - from the blood into the cytosol driven by Na + -dependent transporters in the basolateral membrane.
The Cl - then moves downhill from the cytosol into the lumen through anion channels in the apical membrane. Na +flows passively into the lumen through the tight junctions and paracellular spaces down the electrochemical gradient established by the secondary active transport of Cl - . Water follows by osmosis. |
|
|
What are precursors of digestive enzymes called?
|
Zygomens
|
|
|
What are the 5 zygomens (precursors) of endopeptidases secreted by the acinar pancreas?
|
trypsinogen, chymotrypsinogen, proprotease E, proelastase and kallikreinogen
|
|
|
What are the 2 zygomens (precursors) of exopeptidases secreted by the acini of the pancreas?
|
procarboxypeptidase A and procarboxypeptidase B
|
|
|
What is the lipolytic enzyme precursor secreted by the acini of the pancreas?
|
propancreatic phospholipase A2
|
|
|
As well as the lipolytic enzyme precursor the acini also secretes which lipolytic enzymes?
|
pancreatic lipase and non-specific carboxypeptidase
|
|
|
Which enzymes secreted by the acini is directed against polysaccharides and polynucleotides?
|
Against Polysaccharide - pancreatic amylase
Against Polynuceotides - pancreatic ribonuclease, desoxyribonuclease I and desoxyribonuclease II. |
|
|
How do the zymogens secreted by the pancrease normally activated?
|
The zygomens are normally only activated in the gut lumen. This activation is due to activation of trypsinogen by the brush border protease called enteropeptidase (also called enterokinase).
Active trypsin then activates the other pro-enzymes. |
|
|
The acinar cells also secrete a number of non-enzymatic proteins, name 2 and their function?
|
pancreatic secretory trypsin inhibitor (PSTI) - it is believed to prevent premature activation of zymogens by any active trypsin formed prior to their secretion.
Colipase - a cofactor required for efficient binding of pancreatic lipase to micelles and is required for maximal activity of pancreatic lipase in hydrolysing triglycerides.- It is secreted as a precursor, procolipase. |
|
|
What controls Pancreatic acinar fluid and electrolyte secretion and how?
|
the hormone cholecystokinin and the neurotransmitter acetylcholine. - Both of these act by increasing intracellular free Ca 2+
|
|
|
What do the pancreatic duct cells secrete?
|
a NaHCO 3 rich fluid and low amounts of mucoprotein.
|
|
|
Describe the mechanism of pancreatic ductal NaHCO 3 secretion?
|
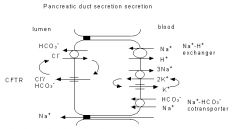
This mechanism is controversial and appears to vary from species to species.
In humans the mechanism of ductal secretion is probably as follows. HCO 3 - is concentrated in the cytosol as a result of the operation of Na + -H + exchangers, Na + -HCO 3 - cotransporters and possibly also H+- ATPases in the basolateral membrane. The HCO 3 - then enters the lumen either through the apical CFTR (the fibrosis transmembrane conductance regulator) anion channel, or in exchange for Cl - through an apical anion exchanger with the Cl - then being recycled across the apical membrane by the CFTR channel. Irrespective of exact mechanism of HCO 3 - transport across the apical membrane, mutations of CFTR which cause the disease cystic fibrosis lead to failure of the duct cells to secrete HCO 3 - |
|
|
What is the stimulus for ductal secretion of NaHCO 3?
|
The stimulus for ductal secretion is the hormone secretin that acts by increasing intracellular cyclic AMP.
|
|
|
True or False? There is a small reserve of functional pancreatic tissue? What is the consequence of this?
|
FALSE - There is a large reserve of functional pancreatic tissue hence under most circumstances fat malabsorption occurs earlier than malabsorption of protein or of polysaccharides.
|
|
|
True or False? Children undergoing subtotal pancreatectomy where 95% of the pancreas is resected often do not have fat malabsorption (steatorrhea)
|
TRUE – there is a large function reserve
|
|
|
In patients with cystic fibrosis what does fat malabsorption occur?
|
not until lipase and colipase secretion are less than 2% of average normal values.
|
|
|
What exacerbates the fat malabsorption in cystic fibrosis?
|
Because pancreatic lipase is strongly pH-sensitive and is only active at alkaline pH, failure of bicarbonate secretion, such as occurs in cystic fibrosis, exacerbates the fat malabsorption produced by reduced secretion of lipase and colipase.
|
|
|
What are the 3 congenital conditions in which protein malabsorption can occur due to pancreatic insufficiency?
|
cystic fibrosis, Schwachman syndrome (congenital hypoplasia of the pancreas) and congenital trypsinogen deficiency.
|
|
|
Describe the difference between the stool in pancreatic insufficiency (eg cystics fibrosis) compared to small intestinal villous atrophy (e.g. coeliac disease)?
|
In pancreatic insufficiency (eg cystics fibrosis) there is undigested trigylcerides (oil or neutral fat) which can be detected in the stool using oil red-O
Whereas in small intestinal villous atrophy (e.g. coeliac disease) the fat digestion is relatively unimpaired but there is impairment of absorption of the products of digestion, fatty acids and monoglycerides so instead fatty acid crystals can be observed microscopically in stool smear preparations. |
|
|
How is starch metabolised?
|
Starch is attacked by salivary amylase and pancreatic amylase, both of which attack linear chains of at least 6 glucose moieties linked by alpha-1,4 bonds.
|
|
|
What inactivates Salivary amylase?
|
It is inactivated by the low pH in the stomach but may digest a significant amount of starch during chewing.
|
|
|
What results following pancreatic amylase digestion of starch?
|
Pancreatic amylase digests starch releasing maltose (glucose- alpha-1 ,4-glucose), maltotriose and alpha-limit dextrins which contain alpha-1,6 bonds that cannot be attacked by amylase.
|
|
|
Following digestion of startch by amylase to Maltose, Maltotriose and alpha-limit dextrins, what is responsible for the next step in digestion?
|
Further digestion requires enzymes that are attached to the brush border of the small intestine. i.e. glucoamylase-maltase, sucrase-isomaltase, lactase and others others.
|
|
|
True or False? Dietary fibre includes plant-derived polysaccharides, such as cellulose, hemi-cellulose and pectin that cannot be digested by human digestive enzymes?
|
TRUE
|
|
|
Dietary fibre includes plant-derived polysaccharides, such as cellulose, hemi-cellulose and pectin that cannot be digested by human digestive enzymes, So how are they utilised by the body?
|
These substances reach the large bowel intact where they are fermented by the colon microflora releasing short chain fatty acids (and gases) that can be absorbed and utilised as sources of energy for the body.
|
|
|
How are proteins digested?
|
Proteins are initially digested by endopeptidase pepsin which is secreted by the chief cells of the gastric mucosa as the inactive precursor pepsinogen.
In the small intestine, proteins are further degraded to oligopeptides by exopeptidases (e.g. carboxypeptidases A and B) and endopeptidases (e.g. trypsin, chymotrypsin, elastases 1 and 2, protease E) which are secreted by the pancreas. |
|
|
Describe the process of lipid digestion?
|
Triglycerides are broken down by pancreatic lipase in the presence of the cofactor, colipase, releasing 2-monoacylglycerol and fatty acids. Cholesterol esters are hydrolysed by the pancreatic enzyme, cholesterol esterase (also called non-specific carboxylesterase). Phospholipids are broken down by phospholipase A2 releasing fatty acids and lysophospholipids.
NOTE - Lipid digestion requires the formation of micelles in the lumen of the small intestine. The formation of micelles requires the presence of bile salts which are secreted by the liver. |
|
|
What are the % of each products of carbohydrate digestion?
|
glucose (80%), fructose (14%) and galactose (5%).
|
|
|
How are glucose and galactose absorbed?
|
Glucose and galactose are transported across the apical membrane of small intestinal villous cells by the Na+ glucose cotransporter, SGLT1, which uses the energy in the Na + gradient to actively transport these monosaccharides into the villous cells.
Then they are transported out of the cell across the basolateral membrane by the glucose transporter GLUT2 (with fructose) |
|
|
How is fructose absorbed following digestion?
|
Fructose is transported across the apical membrane of the villous cells by the glucose transporter GLUT5. Fructose transport by GLUT5 is not coupled to any an ion or other source of energy such as ATP, hence is passive.
Then they are transported out of the cell across the basolateral membrane by the glucose transporter GLUT2 (with glucose and galactose) |
|
|
How are the products of protein digestion (di- and tri-peptides and amino acids) absorbed?
|
Amino acids are transported across the apical membrane of small intestinal villous cells by at least seven transporters that differ in their specificity for particular amino acids and in whether they co-transport the amino acids with Na + or some other ion.
Once inside the cells, the peptides are broken down by cytosolic hydrolases to amino acids. The basolateral membrane of these cells contain at least 5 amino acid transport systems |
|
|
What happens to the MG and Fas once they have entered the enterocytes?
|
In the enterocyte the MG and long-chain (>C16) FA are re-synthesised in the endoplasmic reticulum into triglycerides and bound to specific apolipoproteins (Apo A-I, Apo A-IV, Apo B48) to form chylomicrons which are then secreted and enter the lymphatic drainage system.
Note Medium chain FA (C6-C12), however, do not require re-synthesis to triglycerides and are transported to liver through the portal circulation. |
|
|
What % of wheat flour is gluten?
|
~8%
|
|
|
Why have oats have always been avoided on a GFD although recent research suggests that oats may not contain gluten?
|
Oats produced in Australia are contaminated with small amounts of gluten, derived from wheat and barley grains, and patients are currently advised to avoid them.
|
|
|
The labeling of food as 'gluten-free' is governed by the Australian Food Standards Code, what does it specifiy?
|
They specify that a food must not contain detectable gluten (defined as <0.002% as measured by an ELISA immunoassay), oats or malt 2 , if it is to be labeled 'gluten-free'.
|
|
|
A person on a normal diet consumes about how many grams of gluten per day?
|
10-14 g (10000-14000 mg) of gluten per day.
|
|
|
Why are PPIs given in patients with pancreatic insufficiency taking enzymes?
|
A proton-pump inhibitor also helps to reduce enzyme destruction.
|
|
|
What happens if the jejunum is removed?
|
the ileum adapts to perform jejunal functions, but note - if more than 100 cm of ileum is removed, malabsorption is generally expected.
|
|
|
What happens if the ileum is removed?
|
unlike when the jejunum is removed and the ileum takes over its function, if more than 100 cm of ileum is removed, malabsorption is generally expected.
|
|
|
How is B1 (thiamine) absorbed?
|
Thiamin is absorbed throughout the small intestine either by an active sodium and energy dependent carrier mechanism or by passive diffusion.
|
|
|
How are riboflavin and flavin absorbed?
|
Riboflavin and the flavine nucleotides are absorbed in the proximal jejunum by a saturable transport system following release from food proteins by gastric acidification.
|
|
|
How are Niacin and B-6 absorbed?
|
Niacin and B-6 assimilate in the jejunum by facilitated and/or passive diffusion with B-6 undergoing dephosphorylation prior to absorption.
|
|
|
How is B12 absorbed?
|
Vitamin B-12 is freed from food by gastric acid and then binds to salivary R polypeptides. The latter are then hydrolysed from B-12 by pancreatic proteases, allowing B-12 to bind to intrinsic factor secreted from gastric parietal cells. The B12 Intrinsic factor complex then bond to specific ileal receptors where B-12 is absorbed.
B-12 is then transported through the mucosal cell bound to a carrier protein transcobalamin II. |
|
|
What is required for folate absorption and what inhibits this?
|
Folate requires cleavage from polyglutamates in food by the upper jejunal brush border conjugases prior to absorption.
Conjugase action is impaired in coeliac disease and by drug exposure (salazopyrine, dilantin and alcohol). |
|
|
What are the 4 Fat soluble vitamins?
|
A D E and K
|
|
|
How are fat soluble vitamins absorbed?
|
Fat soluble vitamins are usually incorporated in bile salt micelles prior to absorption by the enterocytes usually in the upper jejunum.
|
|
|
Why do Disorders resulting in malabsorption, including pancreatic insufficiency, cholestasis and villous atrophy can produce fat soluble vitamin deficiencies?
|
Fat soluble vitamins are usually incorporated in bile salt micelles prior to absorption by the enterocytes usually in the upper jejunum.
|
|
|
How is lactose absorbed?
|
lactose is hydrolysed into its component simple sugars galactose and glucose before absorption in the small bowel.
This Hydrolysis of lactose is catalysed by the disaccharidase enzyme lactase, expressed in the brush border of small intestinal (especially jejunal) villus epithelial cells. |
|
|
What is the detection method of malabsorbed lactose (i.e. lactase deficiency)?
|
measurement of breath hydrogen after an oral lactose load.
As mammalian cells do not produce hydrogen in their metabolism, a rise in breath hydrogen about an hour after an oral lactose load represents colonic bacterial metabolism of a substantial amount of unabsorbed lactose. |
|
|
Where is MALT located?
|
Aggregates of non-encapsulated lymphoid tissue are found in the lamina propria and submucosal areas of the gastrointestinal, respiratory and genitourinary tracts.
|
|
|
What antibody type is important for the Humoral immune responses at the mucosal level?
|
IgA.
IgA dimers are actively transported across mucosal membranes and helps prevent entry of infectious microorganisms. |
|
|
What lymphocytes are most prominent in MALT?
|
Lamina propria lymphocytes are predominantly activated T cells, but numerous activated B cells and plasma cells are also present.
|
|
|
What are Intra-epithelial lymphocytes?
|
T cells which display distinct phenotypic features: most express CD8.
One function suggested for IELs is immune surveillance against virus-infected host cells. |
|
|
Describe classical presentation of coeliac disease in childhood?
|
Usually occurs in the toddler age group.
The child presents with anorexia, diarrhoea and irritability and is usually malnourished with pallor and buttock wasting, and has a pot bellied appearance. |
|
|
Describe Osmotic diarrhoea and what causes it?
|
Osmotic diarrhea occurs when too much water is drawn into the bowels.
If a person drinks solutions with excessive sugar or excessive salt, these can draw water from the body into the bowel and cause osmotic diarrhea. Osmotic diarrhea can also be the result of maldigestion (e.g., pancreatic disease or Coeliac disease), in which the nutrients are left in the lumen to pull in water. Also lactase deficiency |
|
|
What sort of physiological diarrhoea is caused by lactase deficiency laxative and pacreatic malabsorption?
|
Osmotic diarrhoea
|
|
|
What are the 2 main differences between Osmotic and Secretory diarrhoea?
|
Osmotic goes on fasting, and there is an osmotic gap in the stool
whereas in secretory diarhoea it is persistant even when fasting and there is no osmotic gap in the stoop |
|
|
Describe Small Bowel Diarrhoea?
|
Large volume, Liquid, Brown or Pale, NO blood or mucous, may contain undigested food
Audible borborygmi and periumbilical pain |
|
|
What are 4 causes of small bowel diarrhoea?
|
coeliac disease, Crohn’s disease, lactase deficiency, drug-induced
|
|
|
Describe large bowel diarrhoea?
|
Small Volume, May contain blood or mucus, straining and tenesmum assosiated
pain – lower abdomen (hind gut) |
|
|
What are 2 causes of large bowel diarrhoea?
|
Ulcerative colitis and ischaemic colitlis
|
|
|
Describe steatorrhea?
|
Bulk, Pale, Oily or Greasy, Very offensive smell, floating and difficult to flush
|
|
|
Name 2 causes of steatorrhea?
|
Pancreatic insuffiency or advanced coeliac disease
|
|
|
Malabospriton of what is lisnged to Distention broborygmi, flatulence and watery stools?
|
disaccharids, monosaccharide and amino acids
|
|
|
What is borborgymus?
|
a rumbling or gurgling noise that occurs from the movements of fluid and gas in the intestines.
|
|
|
What is coeliac disease?
|
an immunologically-mediated disorder of the small intestine caused by dietary exposure to triggering proteins in wheat, barley and rye
This results in villous atrophy which resolves after removal of the offending proteins The manifestations are often non-gastrointestinal |
|
|
describe the genetic aetiology of coeliac disease?
|
HLA DQ2/8 – this allele is present in all cases of coeliac disease but itself is not suffiecient to cause the diease (note 20% of the normal pop are HLA DQ2 +)
90% of coeliacs have HLA DQ2 and the other 10% have HLA-DQ 8 |
|
|
What are the immunogenic protiens assosiated with coeliac disease which are broadly referred to as “gluten”?
|
Wheat – gliadins
Barley – Hordeins Rye – secalins |
|
|
Why cant Testing positive for HLA DQ2/8 CANNOT be used to diagnose coeliac disease?
|
Because while it is neccesary for the disease it is not suffiecient
i.e. 20% of the normal pop are HLA DQ2 + |
|
|
TRUE or FALSE? Testing negative for HLA DQ2/8 excludes coeliac disease?
|
TRUE - Because the allele is neccesary for the disease it is not suffiecient
|
|
|
What is tissue transglutaminase?
|
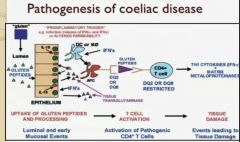
It is a repair enzyme that deamidates glutamine in gliadin to glutamate – (note glutamate is very anitgenic)
This deamindation of gliadin (in wheat) increases the HLA DQ2/8 Presenting cells infinity for gliadin, and this immune response leads to tissue damage |
|
|
What number of cases of of coeliac disease go undiagnosed?
|
for every 1 case diagnosed there are 7 that go undiagnosed.
|
|
|
Name 13 conditions linked to coeliac disease and hence patient with these conditios should be screened?
|
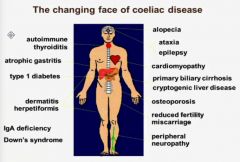
|
|
|
how do you screen/test for coeliac disease and which is the best?
|

Anti-transglutaminase antibody test
|
|
|
What needs to be considered in regards to antibody testing for coeliac disease?
|
5% of coeilacs are IgA deficient (assosiated condition) for IgG testing is required
|
|
|
what other element is essential for coeliac disease diagnosis?
|
small bowel biopsy
|
|
|
Describe the epidemiology of inflammatory bowel disease?
|
Prevalence 1:1000
Developed nations have more cases than developing nations Most common between 15-35 and 50-70 Males = Female s |
|
|
What changes are seen on a small bowel biopsy in a patient with coeliac disease?
|
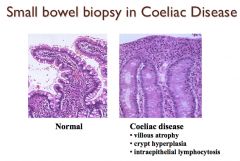
|
|
|
What is ulcerative colitis?
|
It is an inflammatory bowel condition which affects only the mucosa starting at the rectum (dentate line) and travels proximally a variable distance
But note – always confined to the colon |
|
|
Describe Chrons Disease?
|
It is an inflammatory bowel disease which can effect anypart of the GIT (mostly ileum and or colon)
It is a transmural inflammation that effects mucosa and submucosa and hence can result in deep ulcers and fissures It is discontinuous – describe as “skip” lesions Strictures and fistulae are common and granulomas are typical |
|
|
What are the environmental and Host factors that are part of the aetiology of inflammatory bowel disease?
|
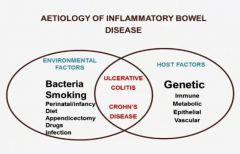
|
|
|
Describe the simplified aeitology of inflammatory bowel disease?
|
Inappropriate immune response to intestinal bacteria in a genetically susepctible host
|
|
|
Describe the genetics assosiated with Chrons Disease?
|
Complex and non-mendelian but there is a 3-20X increase in risk in a 1st degree relative
The genes known to be assosiated with Chrons include NOD2, IL-23R and ATG 16LI |
|
|
In what % of Chrons cases is there bleeding?
|
50%
|
|
|
What is the most common cause on fistulas?
|
Chrons disease
|
|
|
Name the 8 systemic complications of inflammatory bowel disease?
|
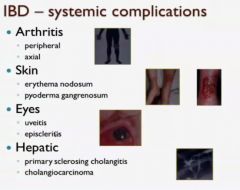
|
|
|
What % of adults have lactase deficeincy?
|
10%
|
|
|
Is iritable bowel disease more or less common in those over 50 years old?
|
less
NOTE – be very cautions in diagnosign anyone over 50 yrs old with IBS as it is usually a red flag |
|
|
What % of ingested fats in triglycerides?
|
95%
|
|
|
describe triglyceride metabolism an absorption?
|

|
|
|
What is the definition of fat absorption?
|
>7% of fat ingested in stool
|
|
|
What enzyme do you test in suspected fat malaborption and why
Also bellow what level does fat abosprtion occur? |
colipase, as it is needed to activate lipase – hence it is a more accurate test than lipase activity
Colipase below 1% is when fat malabsoprtion occures |
|
|
What is ABL?
|
Abetalipoproteinemia, or Bassen-Kornzweig syndrome, is a rare autosomal recessive disorder that interferes with the normal absorption of fat and fat-soluble vitamins from food.
It is caused by a mutation in microsomal triglyceride transfer protein (MTP) resulting in deficiencies in the apolipoproteins B-48 and B-100, which are used in the synthesis and exportation of chylomicrons and VLDL respectively While this disease is rare it is prominent in the lebanses population |
|
|
What is FHBL?
|
Familial Hypobetalipoprotienaemia is an autosomal co-dominant disorder caused by mutations in the Apo-Beta Gene
While this disease is rare it is prominent in the lebanses population |
|
|
What is the presentation of ABL and FHBL?
|
spinocerebellar trait sygns with peripheral neuropathy and ataxia due to vit E defiecency
Their blood film has acanthocytosis |
|
|
How does vitamin A defieceny (cause by fat malabosrption) present?
|
Ocular
– impaired dark adaption (nihgt blindness) xerophthamina – conjunctival dyrness Corneal Dyrness Dermatological - Follicular hyperkeratosis Nuerological Beningn intracranial hypertension (bulging fontanelle) |
|
|
How does vitamin E defieceny (cause by fat malabosrption) present?
|
In premature inflants – haemolytic anaemia
Older children - spinocerebellar trait sygns with peripheral neuropathy and ataxia |

