![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
15 Cards in this Set
- Front
- Back
|
Simple Periodic Trends vs Complicated Periodic Trends
|
Simple = up & down. Based on # of shells.
Complicated = Horizontal. Often takes HCFS into account. |
|
|
Formal Charge
|
= valence electrons - bonds(σ and ρ) - electrons in lone pairs
Each atom in a molecule has it's own formal charge must add up to overall charge of molecule positive formal charges should be on atoms that are less electronegative negative formal charges should be on atoms that are more electronegative |
|
|
Hybridization
|
count each lone pair, single bond, double bond, triple bond as one set of electrons
ex, 4 sets = s p p p = sp³ ex. 2 sets = s p = sp ex. 5 sets = s p p p d = sp³d |
|
|
Molecular Geometry
|
VESPR
sp = linear sp² = trigonal planar sp³ = tetrahedral sp³d = trigonal bipyramidal sp³d² = octahedral |
|
|
Molecular Shape
|
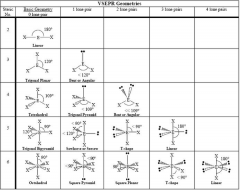
|
|
|
Intramolecular Forces & their strength
|
Chemical Bonds - When electrons are shared between atoms
Strength is dependent on - More electrons shared = stronger bond - smaller atoms = shorter bond length* + exceptions N-N, O-O, F-F are weaker because lone pairs repel Stronger the bond the higher the BDE. It takes energy to break a bond. Endothermic Polar vs. Nonpolar Compare electronegativities for ∆EN If difference = 0 then nonpolar If difference > 0 then polar |
|
|
Intermolecular Forces & their strength
|
Electrostatic attraction of atoms
Strength Depends on - Larger charges equal stronger bonds - Smaller particles = stronger bond* * only important for ionic bonds Stronger Forces have higher BDE Breaking is endothermic |
|
|
Covalent Bonds
|
Formed between atoms with high EN (nonmetals with nonmetals)
Electrons localized between atoms (cannot move) Electrons donated from both atoms Rigid (hard) insulators (because elctrons cannot move) |
|
|
Metallic Bonds
|
metals with metals (low EN)
Electrons delocalized Flexible. (ductile, malleable). Good conductors. |
|
|
Coordinate Covalent Bonds
|
Lone pair shared by electron defficient atom that does not donate
electrons localized Synonym: ligand - chelate: more than one lone pair shared in this manner. Weak bond. Easily dissociated. |
|
|
Ionic Bonds
|
opposite charged ions
localized electrons insulator brittle along cleavage planes smallest atoms with greatest charge (NOT EN) are stronger bonds |
|
|
Dipole Forces
|
Permanently Polar molecules attracted
More polar molecules (high EN difference) Easily cleaved. Short lasting. |
|
|
Dispersion Forces
|
Vanderwahls, induced dipoles, london.
created by deformation from collisions. Creates temporary electrostatic attraction. more electrons = more deformation = stronger forces Very weak. usually not even solids. |
|
|
H bonds
|
N,O,F.
water is best. needs donor (lone pair) and acceptor (N-H, O-H, F-H) to H bond with identical molecule |
|
|
Spectrum of Relative bonds/force strengths
|
Covalent >Ionic>Metalic>Coordinate Covalent>H Bonds>Dipole>Induced Dipole
|

