![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
127 Cards in this Set
- Front
- Back
|
disease
|
dynamic; and interplay b/w injury and its response
|
|
|
T/F. It's not just the injury that causes disease.
|
true; the response may cause disease as well
e.g. renal artery stenosis (-) blood flow >> hypertension |
|
|
sources of variation
|
genetics
age gender situational time laboratory conditions *much more powerful to measure variation against personal baseline |
|
|
etiology
|
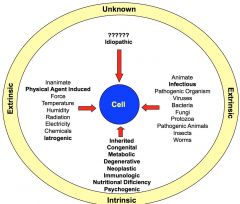
cause of disease
idiopathic- unknown; 90% of HTN iatrogenic- result of medical care |
|
|
factors of pathogenesis
|
time
quantity location morphological changes |
|
|
sign v. symptoms (s&s)
|
sign- objectively observable
symptom- subjectively experienced **pain is both a sign and symptom |
|
|
latent v. prodromal v. acute
|
latent period- b/w onset of injury & first manifestation of s&s
prodromal period- first manifestation of s&s, usually not very specific acute period- severity of s&s is at its highest e.g. jaundice in Hep B patients |
|
|
What happens to disease s/p acute period?
|
It will either resolve itself or enter a chronic phase where severity of s&s is reduced/resolved. However, the disease process is still ongoing. The chronic phase is characterized by period of exacerbation and remission.
|
|
|
subclinical phase
|
disease state is firmly established, however...
>consequences are being well compensated OR > not yet detectable via clinical measures |
|
|
making a diagnosis
|
clinical methods--
s&s laboratory methods-- urianalysis blood analysis (count, chemistry, culture, serology) tissue diagnosis EKG radiography |
|
|
differential diagnosis
|
list all possible diagnoses and begin to narrow until there is only one that matches most/all s&s
|
|
|
factors in maintaining cellular homeostasis
|
cell volume
electrolyte balance pH cell metabolism cell transport |
|
|
How do cells maintain its volume?
|
water balance--
ADH/thirst system osmolyte balance-- renin-angiotensin system (systemic) Na+/K+ ATPase pump (intracellular) |
|
|
water loss and gain
|
if total body weight (+), cell volume will (+)
if total body weight (-), cell volume will (-) gains ++ 1/ water consumed* 2/ water liberated from metabolic processes lost -- 1/ urine* 2/ feces 3/ sweat *indicates primary methods of water +/- under normal conditions; abnormal conditions include diarrhea, excessive sweating, etc. e.g. renal failure: unable to produce urine >> fluid overload |
|
|
ADH/thirst system
|
controlled by monitoring plasma osmolarity @ SF and OVLT >> triggers PVN and SON to produce more ADH when plasma osmolarity increases
ADH promotes H2O retention thirst increases H2O consumption |
|
|
renin-angiotensis system
|
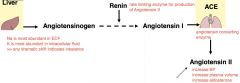
decrease in plasma volume and BP is sensed by JGA granular cell renal baroreceptors >> renin >> angiotensin II
actions of angiotensin II (+) BP (+) plasma volume (+) aldosterone (kidneys, adrenal cortex) >> **increase sodium reabsorption and potassium secretion |
|
|
Na+/K+ pump
|
Na+ more abundant ECF
K+ more abundant ICF *pumps Na+ out / K+ in reliant on ATP, and therefore O2 |
|
|
How much of body weight is total body water (TBW)? Describe the breakdown of TBW and how it relates to body fat.
|
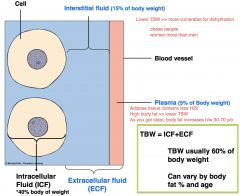
TBW = ICF + ECF
60% = 40% + 20% Greater body fat, less TBW, which increases the vulnerability of dehydration. Women and elderly have more body fat than men and younger people. |
|
|
What makes up ECF?
|
interstitial fluid- 15% of body weight
intravascular fluid (plasma)- 5% of body weight *transcellular space (3rd)- fluid b/w serrous membranes, VERY small part of ECF >synovial joints >peritonial space |
|
|
ECF/ICF composition
|
Na+ more outside
Ca+ more outside* K+ more inside *highly regulated; fluctuation of Ca+ will trigger variety of reactions that can be lethal to cell |
|
|
disrupted fluid movement
|
1/ shift/abnormal distribution b/w ECF and ICF; governed by osmotic balance b/w the two compartments
2/ shift/abnormal distribution b/w interstitial fluid and plasma; governed by starling forces and capillary bulk flow |
|
|
starling forces/capillary bulk flow
|
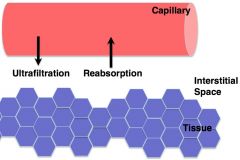
1/ capillary BP- "pushing" favors ultrafiltration
2/ capillary oncotic- "pulling" favors reabsorption 3/ interstitial hydrostatic- "pushing" favors reabsorption 4/ interstitial oncotic- "pulling" favors ultrafiltration |
|
|
causes of edema
|
intravascular/interstitial shift
(+) plasma BP; due to HTN or venous obstruction (-) plasma oncotic pressure **most important, maintains plasma volume; commonly due to diminished production of albumin; other causes are end stage liver failure, starvation >> low BP, high blood viscosity (+) interstitial oncotic pressure due to increased capillary permeability or vascular injury; pores expand so that albumin leaks out and enters interstitial space *lymphatic obstruction; system meant to drain excess interstitial fluid |
|
|
third space accumulation
|
intravascular/interstitial shift
ascites and pleural effusions--difficult to get rid of, requires medical interventions e.g. end stage liver disease, albumin not produced e.g. starvation & malnourishment, albumin not produced *result of low plasma oncotic pressure |
|
|
fluid and electrolyte imbalances
|
ICF/ECF shift
altered Na+, Cl-, and H2O -isotonic alteration -hypertonic alteration -hypotonic alteration potassium balance |
|
|
potassium balance
|
**hyperkalemia/hypokalemia are medical emergencies >> cardiac arrhythmia and sudden death
K+ maintained systematically via angiotensin-renin and Na+/K+ pump other factors that cause ICF/ECF shift-- 1/ pH acidosis, K+ moves out of cell, H+ goes in alkalosis, K+ moves into cell, H+ goes out 2/ insulin is used to treat hyperkalemia (+) insulin, K+ moves into the cell 3/ catecholamines B2 adrenergics, K+ moves into cell A2 adrenergics, K+ moves out of cell |
|
|
isotonic alterations
|
change in TBW accompanied by proportional changes in electrolyte and water, no change in plasma osmolarity
depletion- hemorrhage, severe wound drainage excess- excess IV fluid, hypersection of aldosterone |
|
|
hypertonic alterations
|
ECF osmolarity is elevated
hypernatremia- inadequate water intake, inappropriate administration of hypertonic saline, etc. water deficit- inadequate intake, impaired renal conservation, etc. hyperchloremia- accompanies any excess of Na+ or bicarb deficiency, excess ammonium chloride diuretic (blocks Na+ reabsorption) |
|
|
hypotonic alterations
|
ECF osmolarity is lower than normal
hyponatremia- diuretics, vomiting, diarrhea, burns, dilution (total Na+ does not decline, diluted by H2O) water excess- decrease urine formation, SIADH (syndrome of inappropriate ADH) hypochloremia- accompanies any deficit of sodium or bicarb excess, vomiting, etc. *hypertonic hyponatremia- blood tonicity is elevated by Na+ is reduced due to high blood glucose and cholesterol |
|
|
Which organs primarily regulate acid-base balance?
|
lungs and kidneys
|
|
|
buffer systems
|
pH of fluids are maintained through buffer systems (weak acids and bases that can absorb excess H+ or OH-)
every system has different pH range; by having multiple systems, body is able to increase range some major buffer systems-- bicarbonate hemoglobin proteins phosphate |
|
|
bicarb buffer system
|
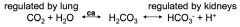
respiratory rate & depth affects PCO2 >> CO2 available for carbonic acid production // rapid effect (mins to hours)
kidneys regulate plasma levels of HCO3- and H+ by controlling HCO3- conservation (reabsorption) and H+ secretion (excretion) // slow effect (hours to days) |
|
|
respiratory acidosis v. respiratory alkalosis
|
respiratory acidosis- hypoventilation, not breathing deeply enough to expel CO2
respiratory alkalosis- hyperventilation, expelling too much CO2 |
|
|
metabolic acidosis v. metabolic alkalosis
|
causes of metabolic acidosis--
(+) noncarbonic acids- ketoacidosis, uremia (build up of nitrogenous waste), ingestion, etc. (-) bicarbonate- diarrhea, renal failure, proximal tubule acidosis (impaired ability to secrete H+, urine is basic but blood is acidic) causes of metabolic alkalosis-- (-) non-carbonic acids- prolonged vomiting, GI suctioning, hyperaldosteronism, diuretic therapy (+) bicarb intake **renal failure causes both metabolic acidosis/alkalosis |
|
|
acid base chart
whos your daddy ??! |

normal pH 7.35-7.45
normal pCO2 35-45 normal pHCO3 22-26 daddy example #1 pH low acidotic pCO2 high favors acidosis HCO3- high favors alkalosis WHAT MATCHES? pCO2, therefore, it is the cause >> respiratory. HCO3- is high because body is trying to compensate. **partially compensated respiratory acidosis; it is partially b/c pH is still abnormal daddy example #2 pH high alkalosis pCO2 high acidosis HCO3- high alkalosis WHO'S YOUR DADDY??! HCO3- >> partially compensated metabolic alkalosis. If pH was normal, it would be uncompensated b/c the system hasn't kicked in yet. |
|
|
cell metabolism
|
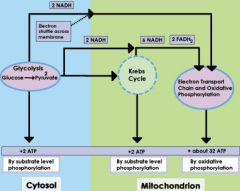
ability of cell to produce enough energy to do work
1/ glycolysis 2/ krebs/citric acid cycle 3/ electron transport chain |
|
|
cell transport
|
passive- simple diffusion, facilitated diffusion, osmosis
active- primary and secondary |
|
|
How do cells react to stress?
|
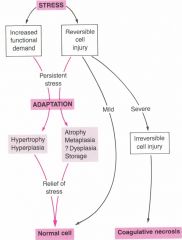
how cells respond to stress depends on the intensity and duration
adaptation usually increases stability, it allows cell survival but alters its functions coagulative necrosis- traumatic cell death |
|
|
mechanisms of cell injury
|
1/ hypoxic injury
2/ free radical / reactive O2 species |
|
|
hypoxic injury
|
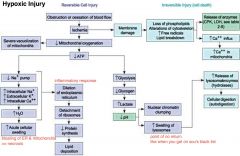
ischemia- impairment of blood flow/O2 to area, e.g. abnormal pulmonary function
anoxic- complete lost of O2 delivery >> tissue infarction re-perfusion can cause further injuries and is done w/ drugs to reduce reactive O2- injury. This may occur due to the layering of injuries... 1/ hypoxic 2/ inflammatory >> neutrophils >> reactive O2- 3/ more reactive O2- during re-perfusion |
|
|
free radical / reactive O2 species
|
free radical- unpaired electron in most outer orbit; hate to be lonely and will steal from others; product of metabolism but we make endogenous agents to offset them
can cause-- lipid peroxidation disruption of polypeptide chains DNA damage by (-) metabolic intake >> (-) radicals >> slow down aging! |
|
|
What are the effects of lipid peroxidation?
|
1/ increased membrane rigidity
2/ decreased activity of membrane enzymes (e.g. Na+ pump) 3/ altered activity of membrane receptors 4/ altered permeability >> water accumulation |
|
|
sources of free radicals
|
metabolism
inflammation air pollution smoking ionizing radiation |
|
|
manifestations of cell injury
|
mostly adaptive, with the exception of dysplasia
each has physiological and pathological forms abnormal cell growth-- atrophy hypertrophy hyperplasia metaplasia dysplasia intracellular digestion failure-- accumulations (water, lipids) hydropic swelling |
|
|
atrophy
|
(-) cell size and function; # stays the same
physio-- utero, childhood development, CNS synaptic pruning patho-- 1/ (-) functional demand or disease (e.g. broken arm) 2/ mild hypoxia 3/ prolonged nutritional deprivation 4/ decreased trophic signal |
|
|
hypertrophy
|
(+) cell size and function; # stays the same
physio-- (+) functional demand, e.g. exercise (+) hormones, e.g. pregnancy, puberty patho-- same stimulation, but it is more than cell can handle; persistent tissue injury, over-secretion of hormones |
|
|
hyperplasia
|
(+) cell #, size stays the same
physio-- (+) functional demand (+) hormone stimulation patho-- abnormal increased demand & inappropriate hormonal secretion, e.g. psoriasis- constant irritation of epidermal area **hypertrophy and hyperplasia occur simultaneously in many cell types; exception is muscle cells because they cannot replicate >> will never encounter L ventricular hyperplasia |
|
|
metaplasia v. dysplasia
|
metaplasia--
chronic injury/irritation; terminally differentiated cells are replace w/ less differentiated cells (not functionally mature) e.g. metaplasia of tracheal epithelium in smokers- simple squamous cell replaces celiated cell and cannot clear airway >> smokers' cough is adaptive to mechanically clear airway dysplasia-- persistent severe injury/irritation; injury to tissue causes precancerous growth (complete derangement, ignore normal control); does not guarantee progression of tumor metaplasia can progress into dysplasia |
|
|
What are some causes of intracellular digestion failure?
|
enzyme deficiency OR alteration in digestive processive
possible pathways 1/ impaired 2/ overloaded 3/ no pathway exists for specific foreign agents |
|
|
tay-sachs disease
|
congenital, lacks enzyme to break down lipid in nervous tissue
|
|
|
What causes fatty liver?
|
alcoholism--
byproduct of ethanol metabolism is acetyl alkahyde which damages liver cells extreme/excessive consumption-- e.g. foie gras |
|
|
hydropic swelling
|
accumulation of water within cell
> already dead or going to die > result of hypoxic injury |
|
|
necrosis v. apotosis
|
necrosis- cell death due to external injury; bloated, exploding, ugly
apotosis- programmed cell death; imploding, collapsing onto self **necrotic cells trigger inflammation, apoptic cells DO NOT! |
|
|
types of necrosis
|
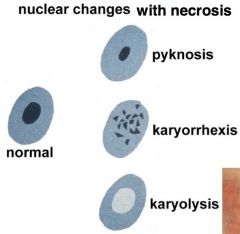
coagulative- zone of dead tissue; cell has not completely lysed & is not completely broken down >> can see cell structure even though cells are dead; e.g. MI
liquefactive- tissue has broken down, cell lacks structural integrity; usually affects CNS caseous- incomplete liquefactive necrosis >> tissue is clumpy and has cheese consistency; e.g. TB fat- breaks down in adipose tissue and combines w/ extracellular Ca+ to form white deposits (calcium soaps); e.g. acute pancreatitis |
|
|
causes of apoptosis
|
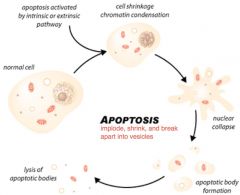
-viral infection
-DNA damage -membrane/mitochondrial damage -cell stress (ER) -induction by immune cells |
|
|
antigen
|
every cell has an identity marker called an antigen
immunity is dependent on the ability to identify cells as 1/ self v. non self 2/ harmful v. nonharmful |
|
|
innate v. adaptive immunity
|
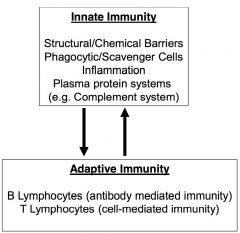
innate- bulk of defense, oldest component and requires no previous experience w/ disease
**components of innate system is needed in order to trigger adaptive immune system adaptive- designed to eradicate disease silently and quickly upon second/third encounter (will get better w/ experience) |
|
|
B lymphocytes v. T lymphocytes
|
B- involved in extracellular infection
T- involved in intracellular infection |
|
|
bacterial v. viral infection
|
bacterial- extracellular; single cell divides and multiplies causing cell and tissue damage when they release harmful substances (e.g. toxic enzymes); can disrupt inflammatory response
viral- intracellular; DNA/RNA surrounded by protein coat, needs host to survive/multiply >>apoptosis >>cancer (cell transformation) >>impair ability to function |
|
|
pluripotent cells
|
1st differentiation of pluripotent cells result in major cell lines--
1/ myeloid cells 2/ lymphoid cells |
|
|
myeloid cells
|
"BEND M2"
basophils eosinophil neutrophil dendritic cell macrophage mast cell |
|
|
neutrophils
|
-found in circulation
-migrate to sites of acute inflammation -first responders, get into interstitial space and dumps reactive O2- species -contain massive lysosomes to help digest pathogens |
|
|
macrophages
|
-neutrophils w/ longer life span
-second responders, release reactive O2- and phagocytose pathogens -can leave site and alert components of immune system -can turn off inflammatory respond and start healing 1/ tissue macrophages are produced in bone marrow during early development and migrate to connective tissue and epithelial barrier (e.g. kuffer cells are macrophages in the liver) 2/ inducible macrophages are found in circulation in form of precursors called monocytes; will become macrophage once it travels to inflammation site |
|
|
dendritic cells
|
-extracellular drainage; identify pathogenic antigen in ECF & trigger adaptive immune response
-found where tissue macrophages are found |
|
|
phagocytes/scavengers
|
macrophages
dendritic cells neutrophils |
|
|
granulocytes
|
**all the phils
-have common precursor -contains large secretory granules where reactive O2- is being produced -found in circulation -inducible -constant supply include neutrophils, basophils, & eosinophils |
|
|
basophils
|
-contain granules packed w/ inflammatory mediators (e.g. histamine)
-has same function as mast cells, but is found in circulation rather than tissues |
|
|
eosinophils
|
-granules have enzymes that degrade membranes and cell wall in ECF
-esp. important in parasitic worm infection and allergic responses |
|
|
mast cells
|
-found in highly vascular tissues and capillary bed
-triggers acute inflammatory response |
|
|
Which cells are found in tissue?
|
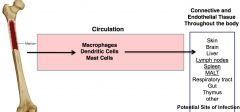
dendritic cells, mast cells, and macrophages
|
|
|
Which cells are found in circulation?
|
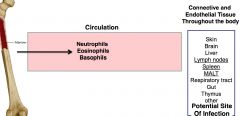
"all the phils & monocytes"
netrophils, eosinophils, basophils, & monocytes these cells are inducible and are constantly produced in case of inflammatory response or systemic infection |
|
|
lymphocytes
|
different surface markers determine identity and functions of different lymphocytes
NK "natural killer" B "bone marrow driven" T helper "thymus derived" T supressor/cytotoxic |
|
|
NK
|
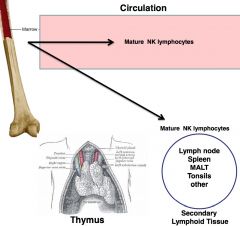
-natural killer cell
-part of innate immune system -similar to T helper cells, but does care about specificity *vital in NEW viral infections |
|
|
B cells
|
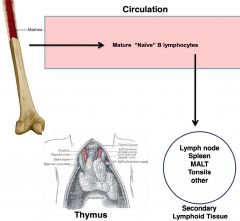
-bone marrow drive; produced and mature there
-IgM and IgD are antibodies; will recognize antigen and carry out antibody mediated response |
|
|
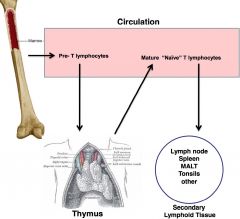
T helper
|
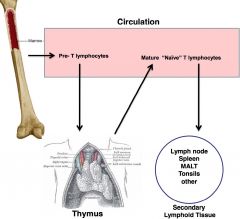
-thymus derived; produced in bone marrow, leave as pre-Ts and mature @ thymus gland when they acquire surface marker that will tell them function and specificity
-activate other cells (B cells and macrophages) CD4 T cells are the *smartest* -can regulate immune reaction and coordinate adaptive immune response |
|
|
T suppressor / cytotoxic
|
CD8 cells are "effectors" and attack pathogens; recognize abnormal host cells infected w/ virus and destroy them
|
|
|
What do NK and T cells have in common?
|
They can recognize transformed cancer cells.
|
|
|
What is the organization of the immune system?
|
There are two overlapping system--
1/ the lymphoid system 2/ the recticuloendothelial system (RES) |
|
|
lymphoid system
|
central lymphoid tissues- where lymphocytes are either produced or mature; sites include bone marrow and thymus
peripheral lymphoid tissues- where cells ultimately reside/activated; sites include lymph nodes, spleen, kidneys, mucosa membrane of respiratory and digestive tract |
|
|
MALT / BALT / GALT
|
MALT- mucus associated lympoid tissue
BALT- bronchialar associated lymphoid tissue GALT- gut associated lymphoid tissue >> these sites represent points of entry in pathogen; barriers are well protected |
|
|
recticuloendothelial system (RES)
|
includes reticular connective tissue, endothelial barriers, & circulatory system
|
|
|
Where can you find pluripotent stem cells?
|
RED bone marrow; yellow bone marrow is mostly adipose tissue =(
|
|
|
inflammation review
|
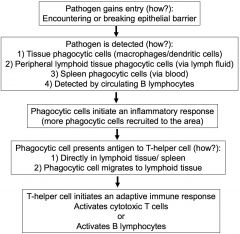
antigen presentation- macrophages and dendritic cells present antigen to SPECIFIC T-helper CD4 cells
|
|
|
lymph node structure
|
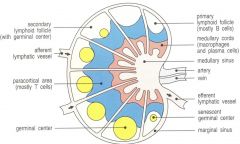
>> afferent lymphatic vessel
>> medullary cords- macrophages and dendritic cells >> paracortical area- T cells >> primary lymphoid follicle- B cells >> germinal center- where different activated T or B cells replicate e.g. spleen consists of 1/ red pulp- traps and breaks down older RBC 2/ white pulp- lymphoid tissue e.g. peyer's patch- associated w/ mucosal barriers; BALT/MALT/GALT |
|
|
cytokines
|
-immune cells communicate and regulate via cytokines, usually named "interleukin __"
-many origins -multiple targets -multiple actions |
|
|
routes of infection
|
1/ pathogen crosses mucus membrane or
2/ pathogen crosses external epithelial e.g. enter via airway, GI or reproductive track e.g. break external barrier; hook worm e.g. vectors; deer ticks (lyme disease), mosquito (malaria) |
|
|
barriers to infection
|
physical- membrane and external epithelial
chemical- lysosomes break down microbial cell wall, gastric pH is extremely acidic microbial- nonharmful bacteria flora creates competition for pathogens |
|
|
innate immunity
|
-barriers to infection (physical, chemical, microbial)
-inflammation -phagocytic cells -plasma protein systems (clotting, complement, kinin) -acute phase response (systematic response to infection e.g. fever, fatigue, & joint ache) -NK cells and interferon (critical for viral infection) |
|
|
inflammatory response
|
1/ vascular
-vasodilation & increased permeability >> redness, heat, edema -delayed vascular stasis (left over blood becomes sluggish, preventing infection from traveling) 2/ cell migration -chemotaxic migration of leukocytes towards cytokines 3/ attack pathogens -phagocytosis or destroyed via extracellular killing -phagocytes carry antigen through lymph fluid to nearest lymph node |
|
|
Which cells can trigger inflammation?
|
mast cells
macrophages |
|
|
cytokines and their effects
|
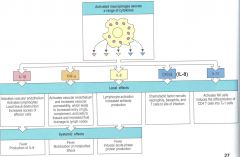
IL-1B
activates mast cells, help stimulate vascular event >> fever, production of IL-6 **secreted and released by macrophages and nitrophils TNF-a strong vascular effect, increases vascular permeability, potent vasodilator >> fever, mobilization of metabolites, shock IL-6 lymphocyte activation, increased antibody production, facilitate w/ adaptive immune response >> fever, induces acute-phase, protein production IL-8 chemotatic factor recruits neutrophils, basophils, and T cells to site of infection IL-12 activates NK cells, induces differentiation of CD4 cells into TH1 cells in case inflammatory response involves a viral infection |
|
|
What can activate mast cells?
|
1/ tissue injury or mast cell injury
2/ IL-1 3/ activated complement 4/ Ig-E mediated mechanism- an antibody that plays key role in allergic reaction (e.g. seasonal allergy) >>sensitization- introduce mast cell to allergen so that body will react against it; first few encounters build up sensitivity |
|
|
actions of mast cells
|
degranulation (immediate response) & synthesis (long-term response)
|
|
|
mast cell degranulation
|
**dump contents of granule into intracellular space
histamine >> vascular effects >> dilation, increased permeability >> exudation IL-8 has neutrophil chemotatic factor >> phagocytosis eosinophil chemotatic factor >> inhibition of vascular effects |
|
|
mast cell synthesis
|
leukotrienes >> vascular effects >> dilation, increased permeability >> exudation
prostaglandins >> vascular effects, pain >> dilation, increased permeability >> exudation -metabolite of arachidonic acid |
|
|
arachidonic acid & co.
|
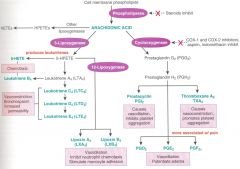
steroids are powerful anti-inflammatory b/c it can block the production of arachidonic acid
cox-1 and cox2 inhibitors block the production of prostaglandin **prostaglandins are more associated with pain; both prostaglandins and leukotrienes enhance migration, adherence and vascular effects |
|
|
cellular events v. vascular events
|
cellular--transmigration
WBC moves fast through blood stream; adhesion is necessary for transmigration to occur--this requires endothelial cells to put out adhesion proteins vascular events--microcirculation includes capillary, metarterioles, arterioles, and venules vasodilation, increased permeability, and transmigration all happen at the capillary beds |
|
|
cell derived chemical mediators summary chart (EVIL)
|
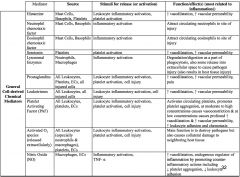
|
|
|
cytokines chart
|

|
|
|
plasma protein systems chart
|

|
|
|
complement system
|
**made up of many circulating plasma proteins that activate a cascade of enzymatic reactions to accomplish three purposes--
1/ promote inflammation 2/ directly kill pathogen 3/ opsonization- tag pathogen for later killing 3 pathways consisting of-- 1/ classical 2/ mb-lectin 3/ alternative >> all three led to C3 convertase "all or none" effect |
|
|
What are three general ways to activate the complement system?
|
1/ the presence of a pathogen
2/ as part of a systemic inflammatory response (liver releases protein that increases likelihood of complement system) 3/ can be activated of adaptive immunity (antibody mediated) |
|
|
complement classical pathway
|
C1 starts the cascade by--
1/ binding directly to pathogen surface 2/ binds to C-reactive protein (CRP) which is already bound to pathogen surface; CRP is released by liver during systemic inflammatory response; its purpose is to bind to C1 in order to increase likelihood of complement 3/ C1 binds to antibody which is bound to pathogen surface |
|
|
complement MB-lectin pathway
|
MB-L is similar to CRP (produced by liver during inflammatory response)
MB-L binds to pathogen surface >> binds to C2 >> C3 convertase |
|
|
complement alternative pathway
|
**only requires presence of pathogen
binding of inactive C3 to pathogen surface >> C3 convertase |
|
|
complement results
|
1/ inflammation
2/ opsonization 3/ directly kills via "membrane attack complex"; activated complement proteins bind together and form channel which gets inserted into pathogen, they go inside, and lysis the bastard |
|
|
kinin system
|
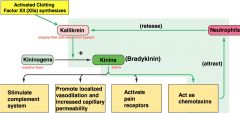
**know this diagram
how is it activated? 1/ XIIa synthesizes kallikrein as a result of vascular damage 2/ when neutrophils arrive, they also release XIIa which amplifies inflammatory response |
|
|
clotting cascade
|
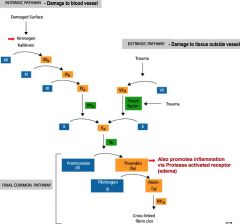
involves 2 activating mechanism
1/ intrinsic pathway- damage to blood vessel 2/ extrinsic pathway- damage to tissue outside vessel >>both lead to final common pathway final common pathway-- fibrinogen are long strands of protein in circulation >> fibrin >> cross linkage traps blood vessels and create clot **key step involves the conversion of prothrombin to active thrombin; it is the final key enzyme before fibrinogen >> fibrin |
|
|
How do the different systems relate?
|
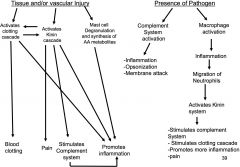
I don't know... I'm starting to think Sally is evil.
|
|
|
innate systemic inflammatory response to infection
|
as infection grows, inflammatory response (+) cytokines which start to enter circulation and binds to key target cells--
1/ brain- effects include fever and sickness (fatigue, aches, & pains) 2/ liver- acute phase, response protein CRP and MB-L enters blood stream & increases complement) 3/ bone marrow- induces production of neutrophils & monocytes |
|
|
endogenous pyrogens actions
|
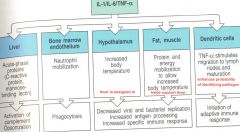
|
|
|
sepsis
|
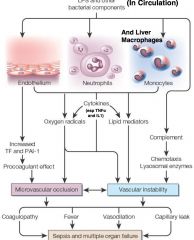
greatly exaggerated inflammatory response; inflammatory response is most effective in tissue bed--it becomes dangerous in circulation
when bacteria leaves tissue and enters the blood stream, it will interact w/ key factors-- 1/ endothelium >> (+) tissue factor and platelet activating factor >> clotting 2/ neutophils >> O2- radicals and lipid mediators >> vascular instability, microvascular occlusion 3/ monocytes 4/ complement *vasodilation and increase in permeability is adaptive on local level but can be destructive at systemic level >> drop in BP, shock >> inappropriate clotting |
|
|
disseminated intravascular coagulation (DIC)
|
*major complication of sepsis
essentially a paradoxical hemorrhage--overactivation of clotting cascade >> no more clotting factors >> unable to clot >> uncontrollable bleeding |
|
|
response to viral infection
|
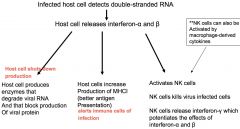
we always produce single strand cytokines
|
|
|
acute inflammation outcomes
|
1/ resolution- complete clearance of injurious stimuli, clearance of mediators and acute inflammatory cells, replacement of injured cells, normal functioning
2/ pus formation (abscess) forms when injured area gets colonized by bacteria; the continued recruitment of neutrophils >> abscess- collection of dead neutrophils 3/ fibrosis- formation of scar tissue w/o abscess; occurs in tissue that cannot regenerate injured or dead cells (e.g. M.I.) **loss of function 4/ progression into chronic inflammation- injury is persistent; active inflammation simultaneously paired w/ attempts to repair the tissue **if chronic inflammation in not interrupted >> healing of scar tissue |
|
|
repair response s/p injury and inflammation
|
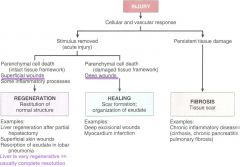
|
|
|
causes of chronic inflammation
|
-persistent infections by microorganisms
e.g. tubercle bacilli, treponema pallidum-syphilis, viruses, fungi, and parasites -prolonged exposure to endogenous and exogenous toxic agents e.g. hyperglycemia, elevated cholesterol, EtOH -autoimmunity e.g. rheumatoid arthritis |
|
|
acute inflammatory response
|
neutrophils >> apoptosis
monocytes >> macrophages >> phagocytosis **macrophages arriving later release cytokines >> growth factor >> new blood vessels and repair of tissue |
|
|
chronic inflammation
|
macrophages get immobilized in tissue or are continuously recruited by persistent injury;
they sense need for on going inflammation and release both sets of mediators >> "frustrated repair" 1/ pro-inflammatory causes further injuries 2/ growth factor that promotes healing **ultimately, the only tissue that survives is fibrotic scar tissue |
|
|
major histocompatibility complex (MHC)
|
**vital in self-recognition
MHCI -found on all cells except RBC -recognized by cytotoxic Tcells and NK cells -display self recognition OR indicate virus infected, non-self, or abnormal self cells MHCII -found in macrophages, dendritic cells, and B cells -recognized by helper T cells (CD4) -involved in antigen presentation and activation of adaptive immune response |
|
|
T/F. MHC proteins are the least diverse among humans.
|
False; most diverse
|
|
|
adaptive immune response overview
|
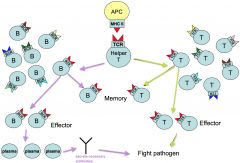
|
|
|
generation of clonal diversity v. clonal selection
|
generation of clonal diversity- initial production of B and T cells that can respond to every possible antigen encountered; mostly occurs in early development but continue throughout life
clonal selection- once we encounter pathogen, we clone the T and B cells that react to antigen in order to fight infection |
|
|
How do we get such diverse B and T cells?
|
germline DNA, genes V, J, and C that code for T-cell receptors are randomly rearranged to create wide variety of combination
randomness of recombination can lead to "auto reactive" T-cells that will bind to self >> as it matures, it will go through clonal deletion; thymus gland will destroy T cell if it binds to one of the self cells along the epithelium **central tolerance- we do not produce B or T cells that will attack our own cells B cells go through identical process in bone marrow |
|
|
antigen presenting cells (APC)
|
*initiate the adaptive immune response
1/ dendritic cells 2/ macrophages 3/ mature but naive B lymphocytes >> this will be mainly done by dendritic cells, whose main purpose is APC >> B lymphocytes will only encounter when in transit from bone marrow |
|
|
T/F. The type of immune response depends on the type of pathogen.
|
true
extracellular pathogens- B-cell mediated response >> antibodies are mobilized into blood stream intracellular pathogens- T-cell mediated response >> effector cells are mobilized to attack pathogen |
|
|
Which cells do intracellular bacteria usually infect?
|
macrophages; bacteria evolved and are able to shut down phagocytic process initiated by macrophages >> bacterial replicate within the macrophages
when these pathogens are recognized, activated T cells differentiate into two subsets-- 1/ TH1- cell mediated 2/ TH2- antibody mediated |

