![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
129 Cards in this Set
- Front
- Back
|
Q: Define the following terms: PHARMACOKINETICS |
PHARMACOKINETICS is the study of how a medication is:
|
|
|
Volume of distribution(Vd) |
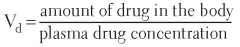
The apparent volume in the body available to contain the drug.
Vd = Dose/Plasma Drug Concentration |
|
|
Is Vd a physiologic value?
|
No
|
|
|
Is Vd an absolute value for any given drug?
|
No
|
|
|
Define the following term:
Clearance (CI) |
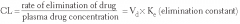
Volume of blood cleared of the drug per unit time;
Total Body Cl = Clhepatic + Clrenal + Clpulmonary + Clother |
|
|
Define the following terms:
Half-life (t₁/₂) |
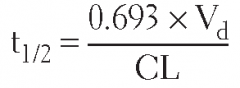
Time required for plasma concentration of drug to decrease by one-half after absorption and distribution are complete;
t₁/₂ = (0.693 × Vd)/(Cl) |
|
|
Define the following terms:
Bioavailability (F) |
The fraction of (active) drug that reaches the systemic circulation/site of action after administration by any route; F = (AUCpo)/(AUCiv), where AUCpo and AUCiv are the extravascular and intravenous areas under the plasma concentration versus time curves, respectively
|
|
|
Define the following terms:
Steady state (Css) |
Steady state is reached when the rate of drug influx into the body = the rate of drug elimination out of the body; Css = plasma concentration of drug at steady state
|
|
|
How much drug is left after two half-lives?
|
25%
|
|
|
How much drug is left after three half-lives?
|
12.5%
|
|
|
During constant infusion, what percent of steady state is reached after one half-life?
|
50%
|
|
|
During constant infusion, what percent of steady state is reached after two half-lives? |
75%
|
|
|
During constant infusion, what percent of steady state is reached after three half-lives?
|
87.5%
|
|
|
During constant infusion, what percent of steady state is reached after four half-lives?
|
94%
|
|
|
Give the equation for the following term:
Infusion rate (K₀) |
K₀= Cl × Css
|
|
|
Give the equation for the:
Loading dose (LD) |
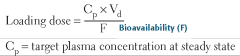
LD = (Vd × CSS)/(F); for examination purposes, F is usually 1
|
|
|
Give the equation for the following term:
Maintenance dose (MD) |

(Cl × Css × τ)/(F), where τ (tao) is the dosing interval
|
|
|
Give the equation for:
Clearance (Cl) |
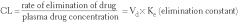
|
|
|
Give the equation for the following term:
Volume of distribution (Vd) |
Vd = (LD)/(CSS)
|
|
|
Give the equation for the following term:
Half-life (t₁/₂) |
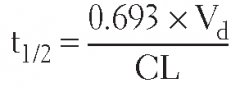
t₁/₂ = (0.693)/(K) or (0.693 × Vd)/(Cl)
|
|
|
What happens to the steady state concentration of a drug if the infusion rate is doubled?
|
Steady state concentration is also doubled; remember that dose and concentration are directly proportional (linear kinetics); Css × K₀/Cl
|
|
|
If there is no active secretion or reabsorption, then renal clearance (Clrenal) is equal to what?
|
Glomerular filtration rate (GFR)
|
|
|
If a drug is protein bound, then Clrenal is equal to what?
|
GFR × free fraction (of drug)
|
|
|
What happens to the LD in patients with impaired renal or hepatic function?
|
Stays the same
|
|
|
What happens to the MD in patients with impaired renal or hepatic function?
|
Decreases
|
|
|
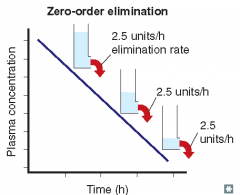
For each of the following statements, state whether it refers to zero-order elimination or first-order elimination?
Rate of elimination is constant, regardless of concentration |
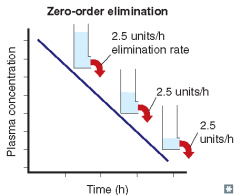
Zero-order elimination
|
|
|
For each of the following statements, state whether it refers to zero-order elimination or first-order elimination?
Plasma concentration decreases exponentially with time |
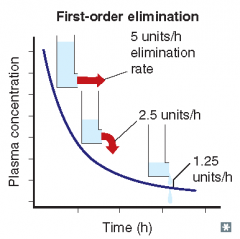
First-order elimination
|
|
|
For each of the following statements, state whether it refers to zero-order elimination or first-order elimination?
Rate of elimination is proportional to the drug concentration |
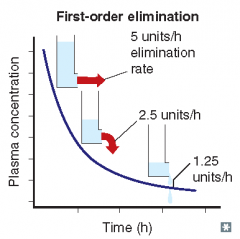
First-order elimination
|
|
|
For each of the following statements, state whether it refers to zero-order elimination or first-order elimination?
Plasma concentration decreases linearly with time |
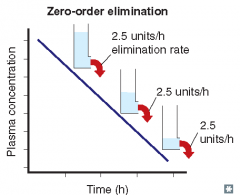
Zero-order elimination
|
|
|
For each of the following statements, state whether it refers to zero-order elimination or first-order elimination?
Rate of elimination is independent of concentration |
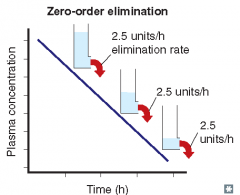
Zero-order elimination
|
|
|
For each of the following statements, state whether it refers to zero-order elimination or first-order elimination?
Rate of elimination is dependent on concentration |
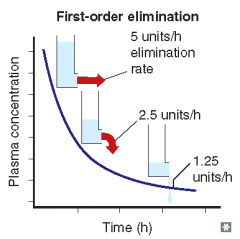
First-order elimination
|
|
|
What are some examples of drugs/substances that undergo zero-order elimination?
|
Acetylsalicylic acid (Aspirin, ASA) at high/toxic concentrations;
phenytoin; ethanol |
|
|
Describe the following types of metabolism:
Phase I metabolism |
Metabolism that generally yields more polar, water-soluble metabolites (may still be active); enzyme activity decreases with patient’s age
|
|
|
Describe the following types of metabolism:
Phase II metabolism |
Metabolism that generally yields very polar, inactive metabolites (renally excreted); enzyme activity does not decrease with patient’s age
|
|
|
Phase I (microsomal) metabolism involves what types of reactions?
|
Oxidation; reduction; hydrolysis (carried out by cytochrome P-450 enzymes)
|
|
|
Phase II (nonmicrosomal) metabolism involves what types of reactions?
|
• Glucuronidation
• acetylation • sulfation • amidation • glutathione conjugation |
|
|
Give examples of drugs that undergo phase II metabolism:
|
Isoniazid (INH), morphine, 6-mercaptopurine, acetaminophen
|
|
|
What are the potential consequences of phase I oxidation reactions with regard to drug activity and elimination?
|
Drug activity may or may not change (no rule, ie, potentially dangerous outcome). Drug elimination is usually increased due to greater water solubility.
|
|
|
What are the potential consequences of phase II reactions with regard to drug activity and elimination?
|
Drug products of phase II reactions are usually inactive and their renal elimination is enhanced.
|
|
|
Where are cytochrome P-450 enzymes found?
|
Smooth endoplasmic reticulum of cells in mainly the liver, but also found in the gastrointestinal (GI) tract, kidney, and lungs
|
|
|
Explain what each type of the following clinical phases in drug development is trying to accomplish?
Phase I |
Safety in healthy individuals; drug pharmacokinetics
|
|
|
Explain what each type of the following clinical phases in drug development is trying to accomplish?
Phase II |
Efficacy in diseased individuals (small scale trials, single- double-blind)
|
|
|
Explain what each type of the following clinical phases in drug development is trying to accomplish?
Phase III |
Efficacy in diseased individuals (small scale trials, single- or double-blind)
|
|
|
Explain what each type of the following clinical phases in drug development is trying to accomplish?
Phase IV |
Postmarketing surveillance (monitored release)
|
|
|
At what point during drug development is an investigational new drug (IND) application filed?
|
Before phase I
|
|
|
At what point during drug development is a new drug application (NDA) filed?
|
After phase III (and before phase IV)
|
|
|
What does the term bioequivalence mean?
|
When comparing two formulations of the same compound, they are said to be bioequivalent to each other if they have the same bioavailability and the same rate of absorption.
|
|
|
What is the first-pass effect?
|
After oral administration, many drugs are absorbed intact from the small intestine and transported first via the portal system to the liver, where they undergo extensive metabolism, therefore usually decreasing the bioavailability of certain oral medications.
|
|
|
How many liters are in each of the following compartments of an average adult human?
Blood |
5L
|
|
|
How many liters are in each of the following compartments of an average adult human?
Plasma |
3L
|
|
|
How many liters are in each of the following compartments of an average adult human?
Total body water (TBW) |
42 L (avg. 70 kg man × 60%. For women 50% of mass [body weight] in kg is body water due to lower lean muscle mass and higher fat content [adipose tissue]).
|
|
|
What is the most common plasma protein that drugs bind to?
|
Albumin
|
|
|
Displacing a drug that is bound to plasma protein(s), for example, albumin, will increase its what?
|
Its free fraction (therefore may possibly increase the risk of toxicity because the plasma concentration of active drug has been increased, yet depending on the drug, an increase in free fraction may actually increase its metabolism because more drug is available to metabolizing enzymes)
|
|
|
For each of the following mechanisms of membrane transport, state if energy is required, if a carrier is required, and if the system is saturable?
Passive diffusion |
No energy required; no carrier; not saturable (proportional to concentration gradient)
|
|
|
For each of the following mechanisms of membrane transport, state if energy is required, if a carrier is required, and if the system is saturable?
Facilitated diffusion |
No energy required; carrier required; saturable
|
|
|
For each of the following mechanisms of membrane transport, state if energy is required, if a carrier is required, and if the system is saturable?
Active transport |
Against concentration/electrical gradient, therefore energy required; carrier required; saturable
|
|
|
The permeation of drugs across cellular membranes is dependent on what drug properties and (local) circumstances?
|
• Drug solubility
• drug concentration gradient • drug ionization • surface area • vascularity |
|
|
Acidification of urine will increase renal elimination of what types of drugs?
|

Weak bases (ionized form of drug, BH⁺) will be trapped in the renal tubules and thus excreted in the urine.
|
|
|
Acidification of urine will decrease renal elimination of what types of drugs?
|
Weak acids (nonionized, HA, form of a drug) can cross membranes.
|
|
|
Alkalinization of urine will increase renal elimination of what types of drugs?
|

Weak acids (ionized, A⁻, form of a drug) will be trapped in the renal tubules and thus excreted in the urine.
|
|
|
Alkalinization of urine will decrease renal elimination of what types of drugs?
|
Weak bases (nonionized, B, form of a drug) can cross membranes.
|
|
|
What agents are used to acidify urine?
|
NH₄Cl; high dose of vitamin C
|
|
|
What agents are used to alkalinize urine?
|
NaHCO₃; acetazolamide
|
|
|
Give an example of a weakly acidic drug:
|

Acetylsalicylic acid (ASA); barbiturates
|
|
|
Give an example of a weakly basic drug:
|

Amphetamines
|
|
|
Give examples of drugs metabolized by each of the following cytochrome P-450 enzymes metabolizes:
CYP 1A2 |
• Caffeine
• ciprofloxacin • theophylline • R-warfarin |
|
|
Give examples of drugs metabolized by each of the following cytochrome P-450 enzymes metabolizes:
CYP 2C9 |
• Ibuprofen
• naproxen • phenytoin • S-warfarin |
|
|
Give examples of drugs metabolized by each of the following cytochrome P-450 enzymes metabolizes:
CYP 2C19 |
Diazepam; omeprazole
|
|
|
Give examples of drugs metabolized by each of the following cytochrome P-450 enzymes metabolizes:
CYP 2D6 |
• Codeine
• dextromethorphan • fluoxetine • haloperidol • loratadine • metoprolol • paroxetine • risperidone • thioridazine • venlafaxine |
|
|
Give examples of drugs metabolized by each of the following cytochrome P-450 enzymes metabolizes:
CYP 2E1 |
Ethanol; INH; acetaminophen (at high doses)
|
|
|
Give examples of drugs metabolized by each of the following cytochrome P-450 enzymes metabolizes:
CYP 3A4 (50%-60% of all therapeutically used drugs are metabolized via CYP 3A4) |
• Alprazolam
• carbamazepine • cyclosporine • diltiazem • erythromycin • fluconazole • itraconazole • ketoconazole • lidocaine • lovastatin • Midazolam • nifedipine • quinidine • simvastatin • tacrolimus • verapamil |
|
|
Give examples of drugs and herbal extracts that generally induce cytochrome P-450 enzymes:
|
• Phenobarbital
• nicotine • rifampin • phenytoin • carbamazepine • St. John’s wort • chronic ethanol consumption |
|
|
Give examples of drugs and foods that generally inhibit cytochrome P-450 enzymes:
|
• Erythromycin
• ketoconazole • ciprofloxacin • quinidine • cimetidine • omeprazole • ritonavir • chloramphenicol • acute alcohol intoxication, grapefruit juice |
|
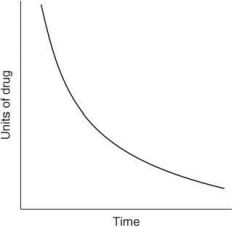
The following graph depicts what type of elimination?
|
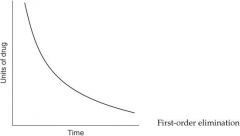
|
|
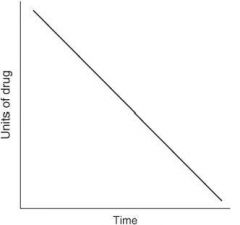
The following graph depicts what type of elimination?
|
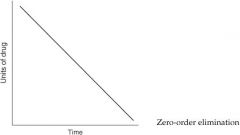
|
|
|
Intracellular volume (ICV) makes up what fraction of TBW?
|
⅔
(ICV = ⅔TBW) |
|
|
Extracellular volume (ECV) makes up what fraction of TBW?
|
⅓
(ECV = ⅓ TBW) |
|
|
Interstitial volume makes up what fraction of ECV?
|
⅔
(interstitial volume = ⅔ ECV) |
|
|
Plasma volume makes up what fraction of ECV?
|
⅓ (plasma volume = ⅓ ECV)
|
|
|
What type of elimination involves a constant fraction of drug eliminated per unit time?
|
First-order elimination
|
|
|
What type of elimination involves a constant amount of drug eliminated per unit time?
|
Zero-order elimination
|
|
|
PHARMACODYNAMICS
|
PHARMACODYNAMICS
|
|
|
Define the following terms:
Pharmacodynamics (PD) |
Field of study that deals with the relationship between plasma concentration of a drug and the body response obtained to that drug; in short, the drug’s effect on the body
|
|
|
Define the following terms:
Affinity |
Ability of a drug to bind to a receptor; on a graded dose-response curve, the nearer the curve is to the y axis, the greater the affinity (affinity deals with drugs acting on the same receptor)
|
|
|
Define the following terms:
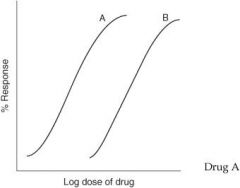
Efficacy |
How well a drug produces a pharmacological response; on a graded dose-response curve, the height of the curve represents the efficacy
|
|
|
Define the following terms:
Potency |
The amount of drug required to obtain a desired effect; on a graded dose-response curve, the nearer the curve is to the y axis, the greater the potency
|
|
|
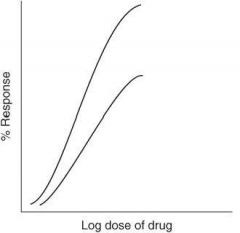
Will the curves for two drugs acting on the same receptor be parallel or nonparallel to each other on a graded dose-response curve?
|
Parallel
|
|
|
Will the curves for two drugs acting on different receptors be parallel or nonparallel to each other on a graded dose-response curve?
|
Nonparallel
|
|
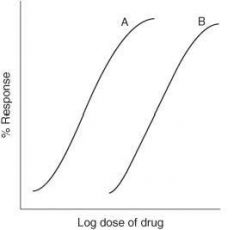
For the graph below, which drug has greater potency?
|
Drug A
|
|
For the graph below, which drug has greater efficacy?
|
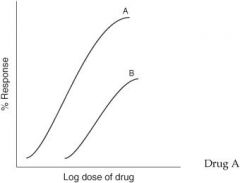
Drug A
|
|
|
Define the following term:
Agonist |
A drug that binds to a receptor, alters its conformation, and activates that receptor’s function
|
|
|
Define the following term:
Full agonist |
An agonist capable of producing a maximal response
|
|
|
Define the following term:
Partial agonist |
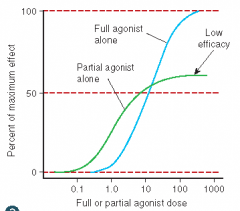
An agonist incapable of producing a maximal response; less efficacious than a full agonist
|
|
|
Define the following term:
Antagonist |
A drug that binds to a receptor and prevents activation of a receptor
|
|
|
Define the following term:
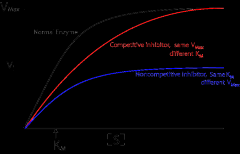
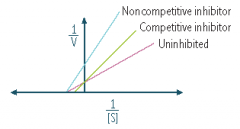
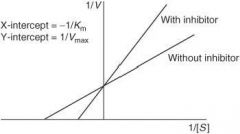
Competitive antagonist |
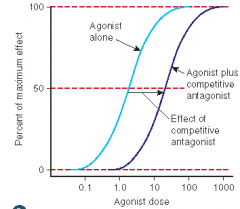
An antagonist that competes with an agonist for the same receptor site; graded dose-response curve will be shifted to the right (parallel) thus decreasing the agonist’s potency (and affinity); maximal response will not be affected
|
|
|
Define the following term:
Noncompetitive antagonist |
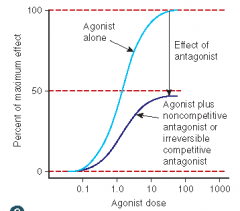
• An antagonist that acts at a different site from the agonist, yet still prevents the agonist from activating its receptor
• potency not affected • maximal response will be decreased • causes a nonparallel shift to the right on a graded dose-response curve |
|
|
What happens when a partial agonist is added in the presence of a full agonist?
|
The response of the full agonist is reduced; partial agonist is acting as an antagonist.
|
|
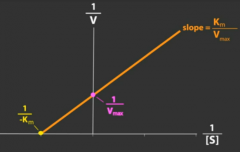
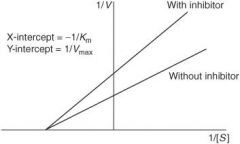
How does a competitive antagonist affect the following?
Affinity (1/Km) of agonist |
Decreased (thus Km will increase as it is inversely proportional to affinity)
|
|
|
How does a competitive antagonist affect the following?
Maximal response (Vmax) of agonist |
No change
|
|
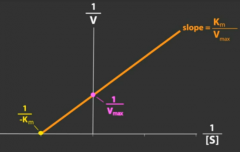
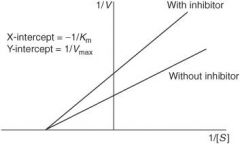
How does a noncompetitive antagonist affect the following?
Affinity (1/Km) of agonist |
No change
|
|
|
How does a noncompetitive antagonist affect the following?
Maximal response (Vmax) of agonist |
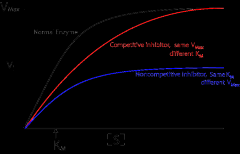
Decreased
|
|
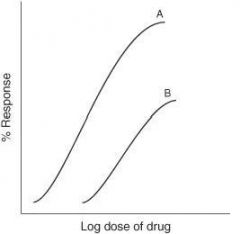
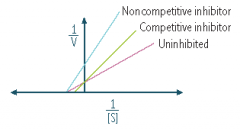
What type of antagonism does the following graph represent?
|

Competitive antagonism
|
|
What type of antagonism does the following graph represent?
|
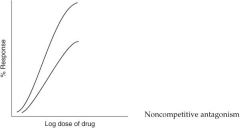
Non-competitive inhibitor
|
|
What type of antagonism does the following graph represent?
|
Non-competitive inhibitor
|
|
What type of antagonism does the following graph represent?
|
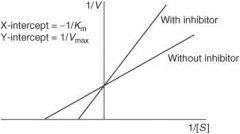
Competitive antagonism
|
|
|
Will increasing the dose of an agonist Yes completely reverse the effect of a competitive antagonist?
|
Yes
|
|
|
Will increasing the dose of an agonist No completely reverse the effect of a noncompetitive antagonist?
|
No
|
|
|
Define the following terms:
Pharmacologic antagonism |
Antagonist and agonist compete for the same receptor site.
|
|
|
Define the following terms:
Physiologic antagonism |
Two different types of agonists acting at different receptors causing opposite responses, therefore, antagonizing each other (acetylcholine [ACh] activating an M receptor to cause bradycardia is antagonized by norepinephrine [NE] acting at a (β-receptor to cause tachycardia)
|
|
|
Define the following terms:
Chemical antagonism |
Response to a drug is antagonized by another compound that binds directly to the effector drug (digoxin is antagonized by digoxin immune Fab[Digibind] which binds directly to digoxin and not its receptor)
|
|
|
Define the following terms:
Potentiation |
When one agonist enhances the action of another compound (benzodiazepines and barbiturates potentiate the effect of gamma-aminobutyric acid (GABA) on its receptor); graded dose-response curve is shifted to the left
|
|
|
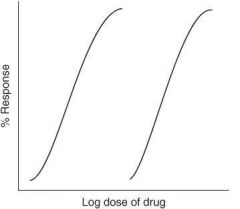
What is a quantal (cumulative) dose-response curve?
|
Curve showing the percentage of a population responding to a given drug effect versus dose of drug given (or log of dose); allows you to visualize intersubject variability regarding drug response in graph form
|
|
|
What is ED₅₀
|
Estimation of the effective dose in 50% of a population; the dose at which 50% of the population will respond to the drug
|
|
|
Can you obtain the ED₅₀ from a graded (quantitative) dose-response curve?
|
No (this curve does not represent a population of individuals)
|
|
|
Can you obtain the ED₅₀ from a quantal (cumulative) dose-response curve?
|
Yes (this curve represents a population of individuals)
|
|
|
What is TD₅₀?
|
Estimation of the toxic dose in 50% of a population; the dose at which 50% of the population will have toxic effects from the drug
|
|
|
What is LD₅₀?
|
Estimation of the lethal dose in 50% of a population; the dose at which 50% of the population will die from the drug
|
|
|
What is therapeutic index (TI)?
|
The relative safety of a drug by comparing the ED₅₀ to either the TD₅₀ or LD₅₀;
the safer the drug, the 'wider' the TI, meaning the TD₅₀ or LD₅₀ is much greater than the ED₅₀; drugs with a 'narrow' TI have their TD₅₀ or LD₅₀close to the ED₅₀ |
|
|
How do you calculate TI?
|
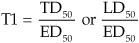
|
|
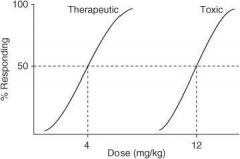
In the following graph, what is the ED₅₀?
|
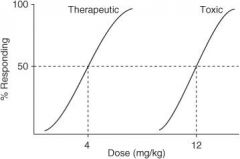
4 mg/kg
|
|
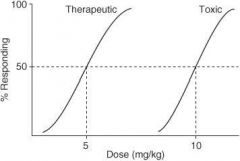
In the following graph, what is the TD₅₀?
|
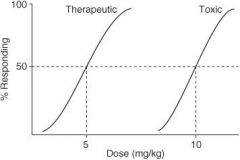
10 mg/kg
|
|
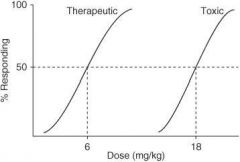
In the following graph, what is the TI?
|
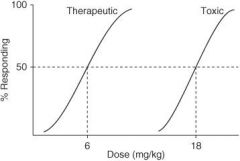
3
|
|
|
What is a narrow therapeutic index (NTI)?
|
Drugs with an NTI usually have a TI less than 2.
|
|
|
List some examples of drugs with an NTI:
|
• Carbamazepine
• digoxin • levothyroxine • lithium • phenytoin • theophylline • valproic acid • warfarin |
|
|
CLINICAL VIGNETTES
|
CLINICAL VIGNETTES
|
|
|
If the ED₅₀ of a particular drug is 100 mg and the LD₅₀ is 1 g, then what is the drug’s therapeutic index (TI)?
|
TI = 10; TI = LD₅₀/ED₅₀
|
|
|
If 100% of a drug is cleared renally, then what would happen to the drug’s half-life if the left kidney was to completely fail?
|
Increase twofold; t₁/₂ - (0.7 × Vd)/(Cl); if only one kidney is 'working' then the clearance has been cut in half thereby doubling the half-life (half-life is inversely proportional to clearance)
|
|
|
A 57-year-old man, that recently underwent a liver transplant 2 years ago, is currently being immunosuppressed with cyclosporin A. The patient asks his primary care physician if he can take over-the-counter St. John’s wort for his 'depressed mood.' How should the patient’s physician respond to this question?
|
The physician should advise against the use of over-the-counter medications. St. John’s wort is an inducer of the cytochrome P-450 enzymes that metabolize cyclosporin A, thereby leading to subtherapeutic serum levels and potentially inducing a graft rejection secondary to inadequate immunosuppression.
|
|
|
A woman who is a long-time Floridian retiree has enjoyed having red grapefruits for breakfast each morning before taking a beach stroll with her friends. Despite her good physical condition she was recently diagnosed with general anxiety disorder and was prescribed Triazolam before bedtime. Does the physician need to learn this patient’s meal habits before prescribing this medication?
|
Yes, consumption of fresh grapefruits and grapefruit juice will inhibit the metabolism of Triazolam leading to overdosing and increased sedation in an elderly patient.
|

