![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
54 Cards in this Set
- Front
- Back
|
Two definitions of cytoskeleton
|
(1) Biochemist definition- the insoluble fraction of cell material that is left after using detergents and centrifugation- chemically undefined
(2) cell biology definition- for eucaryotic cells: made up of three filamentous: actin, microtubules, intermediat filaments. These systems have roles in cell motility, cell shape, mechanical support, and signaling |
|
|
Bacterial flagella
|
A filamentous system which is made up of flagellin, and allows the bacteria to swim by using a rotary motor mechanism which is made by ion gradients
|
|
|
Nematode sperm
|
have a filamentous system made up of MSP (major sperm protein) which allows it to have a crawling movement.
|
|
|
Bacterial flagellum
|
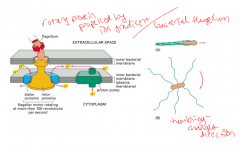
|
|
|
What can filaments do by themselves?
|
Nothing. Need to be anchored, need motor proteins
|
|
|
Actin filament facts
|
They are just beneath the membrane. 5-9nm diameter. 2-stranded, flexible, and polar. Aid in motility and contractility. Actin filament network can move and form lamellipodia protrusion
|
|
|
Intermediate Filaments facts
|
No polarity, tough, ropelike, 10nm diameter, used for structural support. In cytoplasm and form nuclear lamina beneath nuclear membrane
|
|
|
Microtubule Facts
|
25 nm diameter, made of tubulins, rigid, attach to MTOC. polar, used for support, intracell transport, and cell organization, mitosis, cilia and flagella movement.
|
|
|
Mechanical properties of filaments
|
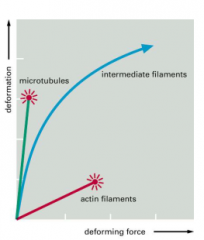
IF are the strongest
|
|
|
Types of actin
|
alpha- found in muscle cells
beta & gamma- non-muscle cells. AA sequence is mostly homogeneous except for the N-terminus |
|
|
Actin structure
|
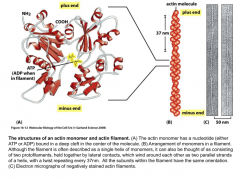
Bound to ATP or ADP. Two stranded polar helix. minus end has an arrowhead structure, plus end is the barbed end.
|
|
|
Actin polymerization
|
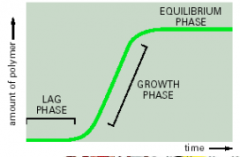
Lag time is the time it takes for monomers to form trimers. The critical concentration is the monomer concentration at the equilibrium state. above it leads to polymerization, below it leads to depolymerization
|
|
|
Treadmilling of actin
|
The critical concentration of the (+) end is lower than the (-) end so in between these concentrations, treadmilling occurs where there is depolymerization at the (-) end and polymerization at the (+) end. This was observed in vivo by using a fluorophor that was activated with light and observed as it moved
|
|
|
Actin regulatory proteins (4)
|
Formin- sits at the (+) end of actin, has two whiskers which attach to profilin.
Profilin- attaches to actin monomers and adds them to the end of an actin filament thymosin- attaches to actin monomers and prevents polymerization and keeps a high monomer concentration inside cells. profilin and thymosin compete to bind to actin monomers. Cofilin- aids in depolymerization of the minus end |
|
|
Arp complex
|
Actin related protein complex of Arp2 & arp3. the complex binds to the side of an actin filament creating a 70 degree angle branch. The complex looks similar to a (+) end and actin monomers bind to it.
|
|
|
3 types of actin organization
|
(1) antiparallel stress fibers- contractile
(2) network- no organization- in cell cortex (3) parallel- same orientation- in filopodia (finger structure). tight packing |
|
|
Actin binding proteins for actin crosslinking
|
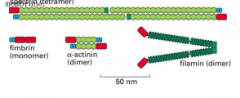
the shorter fibers make a tighter network
|
|
|
What family of proteins regulate actin?
|
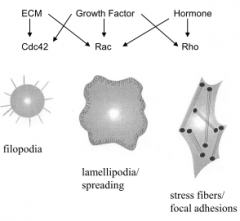
Rho family. (Rho, Rac, cdc42). Rho overexpression leads to stress fibers, Rac overexpression leads to lamellipodia, cdc42 overexpression leads to fillopodia
|
|
|
cell locomotion is caused by...
|
actin polymerization causes cell protrusion, and the interaction between actin & myosin causes cell contraction
|
|
|
Myosin
|
Actin motor protein. Most move towards the (+) end except for Myosin VI. All have head, tail, and neck domains.
|
|
|
Myosin II structure
|
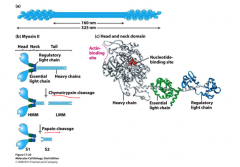
Two heavy chains, two light chains, two walking heads. Form bundles in muscle.
|
|
|
Functions of Myosin I, II, and V
|
Myosin I has one head which attaches to actin and the tail domain can attach to lipids, function: membrane association, endocytosis.
Myosin II- has two heads, used for contraction. Myosin V- has two heads, can carry vesicles and organelles, walks along actin |
|
|
Power stroke cycle of Myosin II
|
(1) Rigor conformation- myosin is rigidly attached to actin. (2) ATP binds to myosin and it releases actin. (3) ATP hydrolysis occurs (4) myosin binds weakly to actin causing Pi to be released. The release triggers a power stroke as the actin is moved, then ADP is released.
|
|
|
Duty time
|
the amount of time myosin is attached to actin. In the myosin II cycle, the duty time is less than 5% because there are many myosin heads attached so they don't all need to be hanging on. For Myosin V, which walks along actin, the heads each need to have a duty cycle of greater than 50% in order for one head to always to be holding on to actin.
|
|
|
Striated muscle structure
|
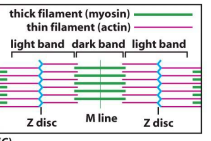
(+) end of actin is anchored to the z disc by CapZ which caps actin and attaches it to z disc. tropomodulin caps the (-) end to prevent depolymerization. As myosin walks towards (+) , sarcomere contracts. Titin is a spring like protein which attaches myosin to the z disc.
|
|
|
Troponin complex
|
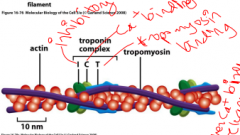
Holds tropomyosin on actin, which blocks the myosin binding to actin. When there is an increase in calcium concentration, tropomyosin is released, myosin is attached, and the muscle contracts.
|
|
|
action potential causes an increase in calcium
|
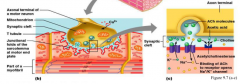
|
|
|
Role of T-tubules
|
T-tubules poke into the plasma membrane and have a voltage gated channel which releases calcium, then the calcium sensitive channel on the sarcoplasmic reticulum membrane opens. This allows signalling to all the muscle fibrils
|
|
|
What regulates smooth muscles?
|
(they don't have striations). Neurotransmitters like acetylcholine and norepinepherine. Hormones like epinephrine and oxytocin. Receptors present on the plasma membrane which NT's and hormones bind to. Can't contract smooth muscle at will.
|
|
|
Smooth muscle filament organization
|
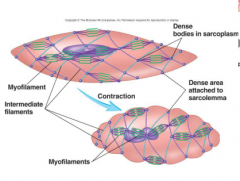
myosins attached to actin are anchored to dense bodies and intermediate filaments. Slower contraction. Have caveolae- indentation sin the sarcolemma which may act as T-tubules.
|
|
|
Regulation pathway of smooth muscle cells
|
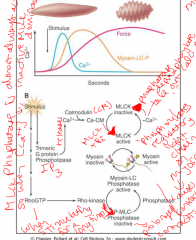
MLCK is almost always active, unless there is a decrease in calcium concentration.
|
|
|
Intermediate Filament structure
|
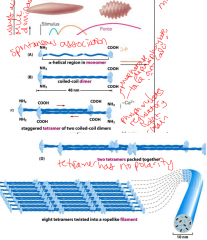
-spontaneously self assemble.
-phosphorylation of heads leads to depolymerization -many different subunits- desmins, lamins |
|
|
IF's aid in what time of cell-cell contact?
|
Desmosomes. They adhere to the inside of the membrane where the cells attach to help hold the cells together
|
|
|
The role of Demin IF's
|
In muscle cells. In striated muscle, hey encircle the z-discs and are cross linked the the plasma membrane and other z-disks. In smooth muscle, desmin anchor dense bodies.
|
|
|
Nuclear Lamina
|
IF's make up the nuclear lamin and aid in cell division. When the lamins are phosphorylated they break up, breaking up the nuclear membrane in prophase. In Telophase, they are dephosphyrlated so that the nuclear membrane is put back together in late telophase
|
|
|
Microtubule structure
|
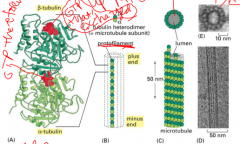
alpha-tubulin and beta-tubulin are always bound to each other, alpha always hangs on to it's GTP. Beta can have GDP or GTP. 13 protofilaments make up a microtubule.
|
|
|
MT organization
|
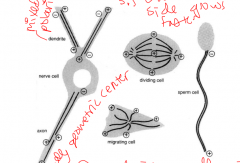
Microtubules grow from the MTOC (microtubule organization center, where the minus end is bound to. The (+) end, which favors polymerization, is at the periphery of the cell. the MTOC surrounds a pair of centriols.
|
|
|
Dynamic instability
|
There is a cycle of growth and dynamic instability at the (+) end of the MT. When the + end has mostly tubulins with GTP, it's called the GTP cap and is stable. When hydrolysis occurs, and GDP is at the top, this is unstable and the protofilaments bend and cause rapid shrinking by depolymerization, called catastrophe. When enough GTP tubulins are added again, the GTP cap is formed again. This happens in cells as well as tredmilling.
|
|
|
Microtubule regulators
|
MAP- stabilizes the (+) end preventing
catastrophin- weakens the (+) end so there is a fastor depolymerization. Katenin- chops up MT's - leads to more depolymerization. Stathmin- binds to tubulin dimers, leading to less monomers available to polymerize |
|
|
Kinesins
|
One family of microtubule motor. Participate in mitotic spiindle dynamics. usually dimers of two heavy chains and two light chains. N-kinesins have the motor end at the N-terminal and are (+) moving. Kinesins with their motor at the c terminal move towards the (-) end.
|
|
|
Dynein
|
The second family of MT motors. Move cargo to the minus end. Also participate in mitotic spindle diynamics. Power beating cilia and flagella. large protein complex with many subunits
|
|
|
Kinesin Structure and movement
|
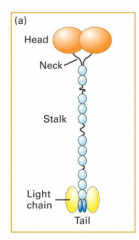
2 heavy chains, 2 light chains. MT and ATP binding sites at the head. cargo-binding site at the tail. Kinesin walks along MT's
|
|
|
What's the difference between kinesin & myosin?
|
Kinesin has a processive motor (walks) where the duty time is greater than 50%. Myosin has a non-processive motor where the duty cycle is less than 5%.
|
|
|
Cilia
|
line the epithelial tissue of the respiratory tract to sweep particulate matter out of the airways. Line the oviduct to push the egg. Non-motile cilia detect signals. Dyneins are responsible for their movement, Cilia beat in unison in a powerstroke motion.
|
|
|
Flagella
|
allow sperm to swim. Are essential for left-right asymmetry during development. Dyneins are responsible for their movement. Make whip like motions and breast stroke motion
|
|
|
Structure of Cilia and Flagella
|

9+2 MT structure. Has arms of dynein attached to the A tubules. The outer dynein has more arms and slide the MT's to cause movement.
|
|
|
Dynein in flagella and cilia
|
A tubule dyneins interact with B tubule of adjacent doublet. cause the MT's to bend and in trypsin, the bonds between MT's (Nexin) are broken and the MT's slide.
|
|
|
Two classes of dyneins
|
(1) Axonemal dyneins- more powerful. localized only in cilia & flagella. Motors power movement. 1-3 heads
(2) cytoplasmic dynein- carries cargo in the cytoplasm to (-) end. Involved in mitosis, 2 heads. |
|
|
Dynactin complex
|

The dynactin complex attaches to cytoplasmic dynein which attaches to a vessicle so that dynein can carry it.
|
|
|
MT's role in metaphase
|
MT's are in an array out of the MTOC on both sides of the cell and the minus ends attach to the kinetochore of the chromosomes in Metaphase. They help to positions the chromotids in the center. Dynein attaches the MT's to the cell wall to create tension on the sides, and kinesin-3 and kinesin-14 can move the MT's away from each other. Kinesin-4,10 help transport the sister chromatids. The balance of push and pull forces of the motor proteins cause the movement of the chromatids
|
|
|
MT's role in Anaphase
|
MT's depolymerize to bring the sister chromatids towards the centrosomes and the poles move away from each other.
|
|
|
Cytokinesis
|
The actin myosin interaction aids in cytokinesis by creating a contractile ring and contracting to split the cells.
|
|
|
How do cells know where to cleave?
|
They know by the ends of the MT's- equidistant away. Spindle poles determine the cleavage not the metaphase plate.
|
|
|
Plectin
|
filament crosslinking protein
|

