![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
31 Cards in this Set
- Front
- Back
|
Matter |
Anything that takes up space and has mass |
|
|
Mass |
the measure of the amount of matter |
|
|
Atom |
the smallest unit of an element that maintains the chemical identity of an element |
|
|
Element |

a pure substance that cannot be broken down and is made up with one atom |
|
|
Compound |
A substance that can be broken down into different substances, made with two or more atoms. |
|
|
Extensive Properties |
How much a substance depends on how much matter is present |
|
|
Intensive Properties |
How a substance does not depend on how much matter present. |
|
|
Physical Property |
A characteristic that means the substance can be observed and measured. |
|
|
Physical Change |
A change on the substance that doesn't change the identity of the substance. |
|
|
Change of state |
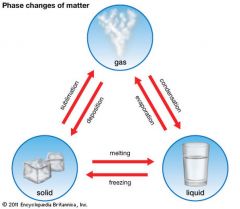
A physical change of a substance from one state to another. |
|
|
Solid |

Definite Volume + Definite Shape |
|
|
Liquid |

Definite Volume but no shape |
|
|
Gas |

No definite shape and volume |
|
|
Plasma |
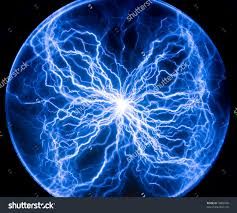
A high-temperature state where the substance loses most of their atoms and electrons. |
|
|
Chemical property |
A substance's ability to undergo changes and transform into other substances. |
|
|
Chemical change |
A change where one or more substances are converted into different substances. |
|
|
Chemical Reaction |
Same as a chemical change |
|
|
Reactant |
Substances that react in a chemical change |
|
|
Product |
Substances made from a chemical change. |
|
|
Mixture |
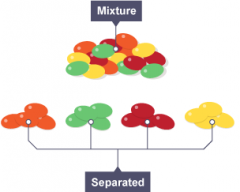
A blend of two different kinds of matter, each retains its identity. |
|
|
Homogenous |
Mixtures that are uniform in a composition, also called solutions. |
|
|
Solution |
A homogenous mixture |
|
|
Heterogenous |
Mixtures that are not uniform in a mixture. |
|
|
Pure substance |
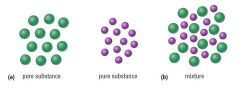
A fixed composition |
|
|
Chemistry |
Study of the composition, structure, and properties of matter. |
|
|
Chemical |
any substance that has a definite composition |
|
|
Group/families |
Vertical columns on the periodic table. |
|
|
Periods |
Horizontal columns on the periodic table. |
|
|
Metal |
an element that is a good electrical conductor and heat conductor. |
|
|
Nonmetal |
an element that is not a good conductor |
|
|
Metaloid |
an element that has some characteristics of both nonmetals and metals |

