![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
10 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
Pure substances |

Elements and/or compounds. Require a chemical process to separate. |
24k gold, silver, carbon dioxide CO2, water H2O. |
|
|
Mixtures |

Combinations of pure substances NOT chemically bonded together. They require a physical process to separate. |
Crude oil, beer, air, 14k gold |
|
|
Elements |
There are approximately 115 known elements in theuniverse. They combine in different combinations to form compounds. |
Krypton(Kr), Carbon(C), Silver(Ag) |
|
|
Compounds |
Compounds have totally different physical sand chemical properties than theelements that make them up.There are millions of different Compounds throughout the world. |
Salt/Sodium Chloride(NaCl) |
|
|
Homogeneus mixtures |
A mixture that is uniform in composition and is the same throughout. Often times referred to as"solutions." |
Examples: tap water, vinegar, Gatorade, beer. |
|
|
Types of solutions |
Gas in gas- air. Gas in liquid- soda. Liquid in liquid- gasoline. Solid in liquid- sea water. Gas in solid- H2 in Pt. Liquid in solid- dental fillings. Solid in Solid- alloys. |
⛽ 🌊 🌪 soda |
|
|
Colloids |
Homogeneus mixtures in which microscopically dispersed insoluble particles between 1 and 1,000 nm in diameter are suspended throughout another substance. Often times they scatter light in something called the Tyndall effect. |
Fog, jello, milk |
|
|
Heterogeneous mixtures |
A mixture that is not uniform in composition and is different throughout. |
Oil & vinegar, fruit salad, concrete, chex mix |
|
|
Suspensions |
Heterogeneous mixtures with particles that have diameters greater than 1,000 nm. Mixtures that say"shake before serving" are usually suspensions. Suspended particles over time settle to the bottom. |
Muddy water, dust in the air, Nail polish |
|
|
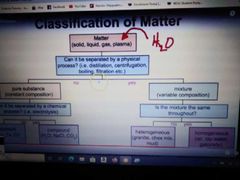
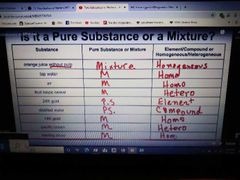
Classification of Matter diagram |
|
|

