![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
78 Cards in this Set
- Front
- Back
|
Name of the number at the bottom of the chemical symbol
|
The atomic number
|
|
|
Name of the number at the top of the chemical symbol
|
The mass number
|
|
|
How do you find the number of electrons with the atomic number
|
It's the same
|
|
|
How is a compound formed
|
When atoms of two or more elements are chemically combined together
|
|
|
Definition of an isotope
|
Different atomic forms of the same element which have the same number of protons but a different number of neutrons
|
|
|
Give an example of a pair of isotopes
|
Carbon-12 and Carbon-14
|
|
|
Which type of bonding is the transferrance of electrons
|
Ionic
|
|
|
Why do elements such as sodium, potassium and calcium like to form ionic bonds
|
They have 1 electron in their outer shell
|
|
|
Which two groups contain elements which are most likely to form ionic bonds by gaining electrons
|
Groups 6 & 7
|
|
|
What kind of structure do ionic compounds have
|
Regular lattice
|
|
|
Why do ions 'stay together' in a lattice structure
|
Because of the strong electrostatic forces of attraction between oppositely charged ions in all directions
|
|
|
Name an example of a compound in a lattice structure
|
Sodium chloride
|
|
|
Name the properties of ionic compounds
|
- High melting points
- High boiling points - Carry electric current when molten - Dissolve easily |
|
|
Why do ionic compounds have high melting and boiling points
|
Because of the strong attraction between the ions. It takes a large amount of energy to overcome this.
|
|
|
Which groups are most likely to form ions
|
Groups 1 & 2 and Groups 6 & 7
|
|
|
Ions have the structure of what
|
A noble gas
|
|
|
Which type of bond is the sharing of electrons
|
Covalent
|
|
|
Draw a diagram for the covalent bond of hydrogen
|

|
|
|
Draw a diagram for the covalent bond of chlorine
|
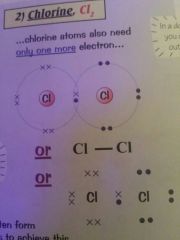
|
|
|
Draw a diagram for the covalent bond of methane
|

|
|
|
Draw a diagram for the covalent bond of hydrogen chloride
|
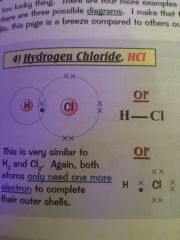
|
|
|
Draw a diagram for the covalent bond of ammonia
|
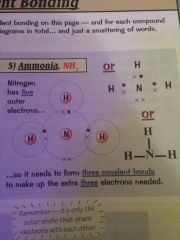
|
|
|
Draw a diagram for the covalent bond of water
|

|
|
|
Draw a diagram for the covalent bond of oxygen
|

|
|
|
Name the two types of substances with covalent bonds
|
- Simple molecules
- Giant structures (macromolecules) |
|
|
Why are the melting and boiling points of simple molecular substances low
|
Because of weak intermolecular forces. The molecules are easily parted from each other.
|
|
|
What happens when simple molecular substances melt or boil
|
Intermolecular forces are broken
|
|
|
What state are most molecular substances in at room temperature
|
Gases or liquids
|
|
|
Why don't molecular substances conduct electricity
|
There are no ions so there's no electrical charge
|
|
|
What is a synonym for giant covalent structures
|
Macromolecules
|
|
|
What is a lattice
|
A giant ionic structure
|
|
|
Why are macromolecules different from lattices
|
Macromolecules have no charged ions
|
|
|
How are atoms bonded together in macromolecules
|
Strong covalent bonds
|
|
|
Features of macromolecules?
|
- high melting & boiling points
- don't conduct electricity |
|
|
3 examples of macromolecules?
|
- Diamond
- Silicon dioxide (silica) - Graphite |
|
|
How many covalent bonds are formed by each carbon atom in diamond
|
4
|
|
|
What is diamond used for
|
Drill tips
|
|
|
What is sand made of
|
Silicon dioxide
|
|
|
In graphite how many covalent bonds are formed by each carbon atom
|
3
|
|
|
What do the three covalent bonds formed by each carbon atom in graphite create
|
Layers which are free to slide over each other
|
|
|
What features are created in graphite by the layers formed
|
Makes it soft and slippery
|
|
|
How does a pencil work
|
The layers are held together so loosely in graphite that they can be rubbed off on to paper
|
|
|
Why can layers be rubbed off on to paper in graphite
|
Because there are weak intermolecular forces between layers
|
|
|
Why is graphite a good conductor of heat and electricity
|
Each carbon atom has one delocalised electron which conducts it
|
|
|
What are the properties of metals due to
|
The sea of free/delocalised electrons
|
|
|
Where do the delocalised electrons come from
|
The outer shell of every metal atom in the structure
|
|
|
Why are metals good conductors of heat and electricity
|
Because the electrons are free to move throughout the structure
|
|
|
Why are atoms in metals held together in a regular structure
|
There are strong forces of electrostatic attraction between the positive metal ions and the negative electrons
|
|
|
What does the sea of free electrons allow to happen in metals
|
The layers of atoms can slide over each other allowing metals to be bent and shaped
|
|
|
Which are harder; pure metals or alloys
|
Alloys
|
|
|
What is an alloy
|
A mixture of two or more metals
|
|
|
Why are alloys harder than pure metals
|
Different elements have different sized atoms. The new metal atoms will distort the layers of metal atoms making it more difficult for them to slide over each other.
|
|
|
Name the 4 types of structures
|
- Giant ionic
- Simple molecular - Giant covalent - Giant metallic |
|
|
What do smart materials do
|
Behave differently depending on the conditions
|
|
|
Give an example of a smart material
|
Nitinol, a shape memory alloy
|
|
|
State the properties of nitinol
|
- When cool it's bendable and you are able to twist it like rubber
- When heated above a certain temperature it goes back to a remembered shape |
|
|
Give two examples of uses for nitinol
|
- Glasses frames, if accidentally bent they can be put in a bowl of hot water and they'll jump back into shape.
- Dental braces, it warms in the mouth and tries to return to a remembered shape and pulls the teeth with it. |
|
|
What are nanoparticles
|
Tiny particles, 1-100 nanometres across
|
|
|
How many atoms do nanoparticles contain
|
Roughly a few hundred
|
|
|
Nanoparticles include what
|
Fullerenes
|
|
|
What are fullerenes
|
Molecules of carbon, shaped like hollow balls or closed tubes.
The carbon atoms are arranged in hexagonal rings. |
|
|
What is a nanotube
|
Fullerenes joined together.
They're tiny hallow carbon tubes, a few nanometres across. |
|
|
Which type of bond in nanotubes make them strong
|
Covalent
|
|
|
Give an example of an object in which nanotubes are used
|
Used to reinforce graphite in tennis rackets
|
|
|
Using nanoparticles is known as
|
Nanoscience
|
|
|
List possible uses for nanoparticles
|
- Catalysts due to large surface area to volume ratio
- Highly specific sensors to test water purity - To make stronger, lighter building materials - Cosmetics (sun cream, deodorant) - Electric circuits - Artificial joints - Gears |
|
|
Explain the idea behind nanomedicine
|
Tiny fullerenes can be absorbed more easily by the body than most particles. Drugs can then be delivered right into the cells where they're needed.
|
|
|
Why can nanotubes be used in electric circuits
|
They conduct electricity
|
|
|
Why can fullerenes be used to aid artificial joints and gears
|
Lubricant coatings are being developed using fullerenes. They reduce friction a bit like ball bearings so can be used in artificial joints etc.
|
|
|
What determines the properties of plastics
|
Forces between molecules
|
|
|
What holds the atoms together in long chains in plastics
|
Strong covalent bonds
|
|
|
Describe polymers with weak forces
|
Individual tangled chains, held together by weak intermolecular forces which are free to slide over each other.
|
|
|
Describe polymers with strong forces
|
Strong intermolecular forces between polymer chains called crosslinks, that hold the chains together.
|
|
|
Why can thermosoftening plastics be melted
|
They don't have cross-linking between chains. The forces between them are really easy to overcome.
|
|
|
Why can't thermosetting plastics be melted
|
They have crosslinks which hold the chains together in a solid structure.
|
|
|
Give examples of two types of polythene made using different conditions
|
- Low density polythene
- High density polythene |
|
|
How is LDPE made & what are its properties
|
By heating ethene to about 200 degrees C under high pressure.
Flexible, used for bags and bottles. |
|
|
How is HDPE made & what are its properties
|
Made at a lower temperature than LDPE and pressure (with a catalyst).
More rigid than LDPE, used for water tanks and drainpipes. |

