![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
42 Cards in this Set
- Front
- Back
|
Which of the following statements about hypothesis is false? A. A hypothesis must be testable in a way that is replicable by others. B. A hypothesis is an explanation for a natural phenomenon. C. A hypothesis should be created when the outcome for its test is known. D. A hypothesis is an educated guess about why something happens |
C. |
|
|
Choose the true statement A. A hypothesis always leads to a scientific theory. B. A scientific theory summarizes a hypothesis that has been supported with repeated testing. C. To be called a hypothesis, an explanation must be tested by multiple independent researchers. D. The theory is established first followed by the hypothesis. |
B |
|
|
Scientific method |
The process that lies at the center of scientific inquiry |
|
|
Theory |
Is a set of tested hypothesis that give an overall explanation of some part of nature. |
|
|
Chemistry |
The science that deals with the materials of the universe and the materials that these materials undergo. |
|
|
Matter |
The stuff of which the universe is composed, the two characteristics are it has mass and occupies space. |
|
|
Three stages of matter |
Solid, liquid, gas |
|
|
Solid |
Rigid, has a fixed shape and volume |
|
|
Liquid |
Has a definite volume but takes the shape of its container |
|
|
Gas |
Has no fixed volume or shape; takes the shape and volume of its container |
|
|
Physical properties |
Boiling point, melting point, diamond is hard, a metal wire conducts electric current, |
|
|
Chemical properties |
Ability to format new substances, wood burning, rusting of steel, digestion of food, growth of grass in our yard. |
|
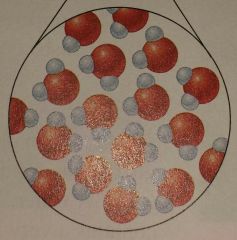
What state of matter is this? |
Liquid |
|
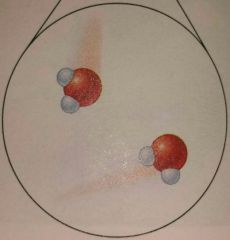
What state of matter is this? |
Solid |
|
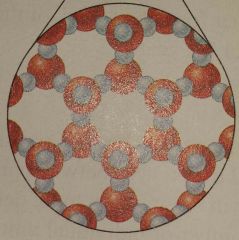
What state of matter is this? |
Gas |
|

|

|
|
|
Classify each as a chemical or physical change A. Milk turns sour B. Wax is melted over a flame and them catches fire and burns |
A. Chemical B. Chemical |
|
|
Elements |
Cannot be broken down into other substances by chemical means. |
|
|
Compounds |
Substances that have the same composition no matter where we find them. They are made out of elements and therefore can be broken down into elements through chemical changes. Example, water. |
|
|
Mixture |
Can be defined as something that has variable composition. Ex, wood is a mixture, wine is a mixture, coffee, and groundwater. |
|
|
Pure substance |
Always has the same composition. Pure substances are either elements or compounds. Pure water is a compound. Groundwater is a mixture. |
|
|
Solution |
Another word for mixture |
|
|
Heterogeneous mixture |
Contains regions that have different properties from those of other regions. Ex, when we pour sand into water, there will be an area that's mostly water and one that's mostly sand. |
|
|
Homogenous mixture |
Is the same throughout. Ex, when we pour salt into water, all the regions of the mixture have the same properties. |
|
|
Identify each as pure substance, homogenous mixture, or heterogeneous mixture. A. Gasoline B. Stream with gravel at the bottom C. Air D. Brass E. Copper metal |
A. Homo B. Hetero C. Homo D. Homo E. Pure |
|
|
Distillation |
The process of boiling water to turn it to steam, and it condenses to pure water |
|
|
Is there a difference between a homogenous mixture of hydrogen and oxygen In a 2:1 ratio and a sample of water vapor? |
Yes, a mixture of hydrogen gas and oxygen is composed of 2 types of molecules. A sample of water vapor contains only 1 type of molecule, h2o. Even if there is 2 times as much hydrogen gas as oxygen gas in the sample, the molecules of h and o2 are distinct, whereas the 2h and o atoms in water are connected(bonded). |
|
|
Are all physical changes accompanied by chemical changes? Are all chemical changes accompanied by physical changes? Explain. |
No. Physical changes are not necessarily accompanied by chemical changes. However chemical changes are always accompanied by physical changes. |
|
|
_______ has a fixed shape and volume. |
Solid |
|
|
Matter in the ______ state has no shape and fills completely whatever container holds it. |
Gas |
|
|
Which of the following represents a chemical change? A. Frying an egg B. Diluting orange juice C. Sublimation of dry ice D. Molding melted silver |
A. Frying an egg |
|
|
How can you tell that a physical change has taken place? |
The properties of the starting and ending materials are the same. |
|
|
What is the difference between a hypothesis and a scientific theory? |
A hypothesis is a tentative proposal and a theory is a tested proposal. |
|
|
What is the difference between a scientific theory and a natural law? |
A theory explain s behavior and a law states a measure able relationship. |
|
|
Proton (p+) |
Positively charged subatomic particle found In the nucleus of the atom. |
|
|
Neutron (n) |
Neutral subatomic particle found in the nucleus. |
|
|
Electron(e-) |
Negatively charged subatomic particle found outside the nucleus. |
|
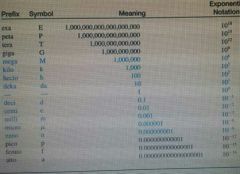
|
Memorize the blue part |
|
|
If the number is a whole number move the decimal to the____ |
Left |
|
|
If the number is a decimal move the decimal point to the _____ |
Right |
|
|
If the decimal is moved to the right the exponent is_____ |
Negative |
|
|
If the decimal is moved to the left the exponent is _____ |
Positive |

