![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
22 Cards in this Set
- Front
- Back
|
key advantage offered by flow cytometry over immunohistochemisty?
|
flow cytometry performs multiparametric analysis on individual cells; the key application of this analysis is identifying dual expression of CD antigens on a cell...CD5 and CD19 together in CLL/SLL
|
|
|
outline the basic priniciple of flow cytometry?
|
cells from a specimen are suspended in fluid; injected single file into the center of a flowing stream of fluid which passes through a quartz capillary tube; the cells are illuminated via laser beams which yeilds information about the size and complexity; fluorochromes may be attached to the cells...give data about the cell surface markers
|
|
|
compensation coefficient?
|
the contribution of other fluorochromes to the signal are subtracted to isolate the fluorescence from the desired fluorochrome; the subtraction is termed compensation; fluorochromes may vary from lot to lot....run controls
|
|
|
lymphadenopathy work up
peripheral blood=EDTA tube marrow aspirate= heparin tube lymph node=RPMI ok? |
yes; either blood or bone marrow may be anticoagulated with EDTA, heparin or acid citrate dextrose; tissue is tranported in RPMI
|
|
|
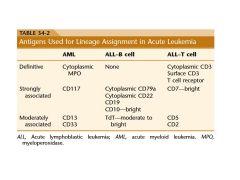
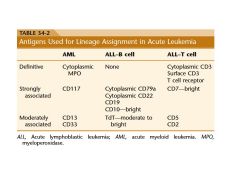
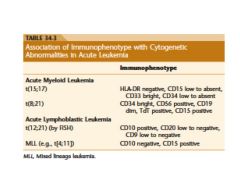
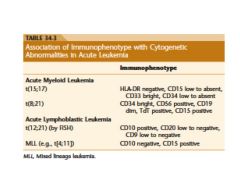
four antigens found on blast in acute leukemia
|
CD34, CD117, CD133, and TdT are the key markers for blast
|
|
|
diagnosis of acute leukemia?
|
20% blast are required for the diagnosis of acute leukemia
|
|
|
cell surface marker profile in mycosis fungoides/sezary syndrome?
|
CD4 positive, loss of CD7
|
|
|
CLL , poorer prognosis?
|
CD38
ZAP 70 |
|
|
what two key cell surface markers are used to identify plasma cells?
|
CD38, CD138
|
|
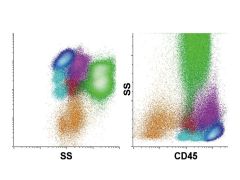
|
CD45 versus side scatter. The display of CD45 versus side scatter is increasingly used in the clinical laboratory as a starting point for the identification of white blood cell populations. Data are commonly displayed with side scatter on a logarithmic (left) or linear (right) scale. Major white cell populations colored are lymphocytes (blue), B cells (light blue), monocytes (purple), maturing neutrophils (green), blasts (red), basophils (dark purple), and maturing erythrocytes (orange).
|
|
|
|
|
|
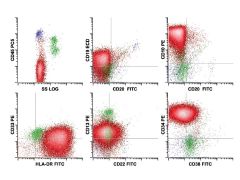
5 yog with fatigue and elevated WBC; population of interest in red; DX?
Precursor B cell lymphoblastic leukemia/lymphoma (acute lymphoblastic leukemia). The neoplastic cell population (red) in comparison with normal immature B cells shows abnormal expression of B cell–associated antigens CD19 (dim), CD22 (very dim), and CD10 (bright), along with aberrant expression of CD45 (absent), CD34 (bright), and CD38 (dim). In addition, the low-level expression of myeloid-associated antigens CD13 and CD33 is seen—a feature frequently seen in this disorder and not suggesting myeloid differentiation by itself. The cells have low side scatter, as is typical of lymphoid blasts. |
|
|
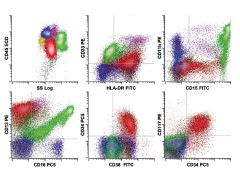
Acute myeloid leukemia with differentiation. The neoplastic cell population consists of blasts (red) showing abnormal expression of the myeloid-associated antigens CD33 (dim), CD13 (bright), and CD15 (dim partial) in association with immature antigens CD34 and CD117. The blasts do not express the more mature neutrophilic antigens CD11b and CD16, and are typical of the relatively immature forms seen in many cases of acute myeloid leukemia. Background maturing granulocytes are present (green) that are normal and are not part of the leukemic population.
|
|
|
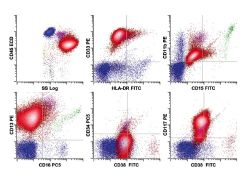
Acute promyelocytic leukemia. The neoplastic cell population consists of promyelocytes (red) showing abnormal expression of the myeloid-associated
antigens CD33 (bright) and CD13 (intermediate), with high side scatter indicating abundant cytoplasmic granularity. The population lacks expression of CD34 and HLA-DR, antigens commonly present on true myeloid blasts, and retains expression of CD117, as is seen on a subset of normal promyelocytes. However, in contrast to normal promyelocytes, abnormal cells lack significant expression of CD15, a characteristic and common abnormality in this disorder. |
|
|
|
|
|
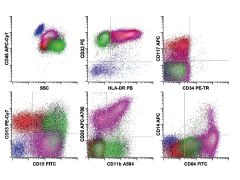
Acute myelomonocytic leukemia. The neoplastic cells consist of a relatively small population of blasts (red) with a larger population of cells showing monocytic differentiation (violet). The blasts show abnormal expression of CD33 (bright), CD13 (intermediate), HLA-DR (intermediate), and CD117 (intermediate) without CD34. The monocytic differentiation is reflected in the acquisition of early monocyte antigens CD64 (bright) and CD36 (intermediate to bright), along with other more mature myelomonocytic antigens CD15 (intermediate) and CD11b (low to intermediate), without significant acquisition of the mature monocyte antigen CD14 (absent) or marked gain in the expression of CD45, as is seen in mature monocytes. This finding suggests differentiation to the promonocyte stage, a population usually included in morphologic blast counts. In addition, a lesser degree of neutrophilic differentiation (green) is present.
|
|
|
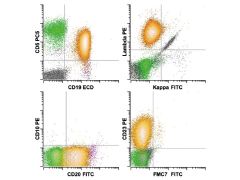
Chronic lymphocytic leukemia/small lymphocytic lymphoma. The neoplastic B cell population (gold) shows abnormal expression of the B cell antigens CD19 (intermediate) and CD20 (low) with surface λ light chain expression (low) and coexpression of CD5 and CD23 (intermediate). The combination of CD5 coexpression, low-level light chain restriction, and low-level CD20 and CD23 without FMC7 is diagnostic for this particular disorder. The important differential is seen with mantle cell lymphoma
|
|
|
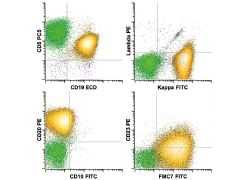
Mantle cell lymphoma. The neoplastic B cell population (gold) shows expression of B cell antigens CD19 (intermediate) and CD20 (intermediate) and κ light chain restriction (bright), along with coexpression of CD5 (intermediate) and FMC7 (intermediate) with only low CD23. The overall pattern of antigen expression differs from that seen in CLL/SLL
(see Fig. 34-17) and suggests mantle cell lymphoma. Definitive diagnosis would require demonstration of t(11;14) by FISH or cyclin D1 overexpression by immunohistochemistry. |
|
|
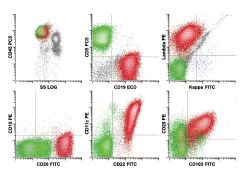
Hairy cell leukemia. The neoplastic B cell population (red) shows expression of B cell antigens CD19 (intermediate), CD20 (bright), CD22 (bright) and
surface λ light chain restriction (intermediate) with aberrant expression of the adhesion molecules CD11c (variably bright), CD103 (intermediate), and CD25 (intermediate). This immunophenotype is diagnostic for hairy cell leukemia. Note the absence of monocytes by CD45 versus side scatter, a characteristic finding in hairy cell leukemia. |
|
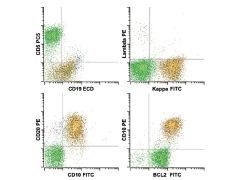
|
Follicular lymphoma. The neoplastic B cell population (gold) shows abnormal expression of the B cell antigens CD19 (dim), CD20 (intermediate), and surface κ light chain restriction (intermediate) with coexpression of the follicle center–associated antigen CD10 (intermediate). In addition, the neoplasm shows overexpression of cytoplasmic Bcl-2 due to the presence of the t(14;18) characteristic of follicular lymphoma. Overexpression of Bcl-2 by itself is not diagnostic of follicular lymphoma, but its combined expression with CD10 is much more specific, as normal CD10-positive follicular B cells would be expected to be negative.
|
|
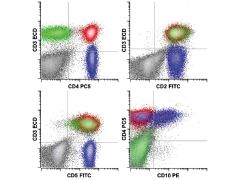
|
Angioimmunoblastic T cell lymphoma. The neoplastic T cell population (blue) shows expression of the T cell–associated antigens CD2 (intermediate) and CD5 (intermediate), along with the helper T cell antigen CD4, but without surface CD3. In addition, the neoplastic cells coexpress CD10. The composite immunophenotype is characteristic of that seen in angioimmunoblastic T cell lymphoma.
|
|

|
Classic Hodgkin lymphoma. A discrete population of cells is identified that differs from normal lymphocytes and macrophages, having expression of CD15,
CD30, high CD40, high CD71, and high CD95. This immunophenotype is typical for classical Hodgkin lymphoma. Note that these cells commonly show increased autofluorescence that appears as apparent low positivity for FITC-labeled reagents (CD64 in the current example). Also note that Hodgkin cells commonly are rosetted by T cells, giving apparent positivity for CD5 and CD45 ( |

