![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
3 Cards in this Set
- Front
- Back
|
The specific latent heat of fusion of a substance is 1.8kj/kg.How much energy is required to melt the 2.2kg of this substance? |
Answer= L=1.8kj/kg = 1800j/kg M=2.2 kg E= 2.2x1800=38600 |
|
|
An object of mass 5kg requires 16kj of energy to melt it.What is it’s latent heat of fusion? |
Method: 16000 divide 5kg=3200 |
|
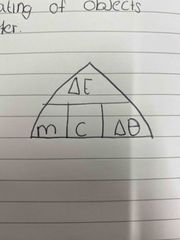
|
What causes the pressure of a gas on a surface?-The force of the gas particles hitting the surface. |

