![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
20 Cards in this Set
- Front
- Back
|
How can energy change during a reaction? |
Energy change occur either through heat transfer or work or both. |
|
|
Describe the flow of thermal energy between two objects at different temperatures |
Thermal energy flows from a hotter place to a colder place until they reach the same temperature. |
|
|
What are the difference between an endothermic reaction and exothermic reaction? |
an exothermic reaction releases heat and an endothermic reaction absorbs Heat |
|
|
Give examples of an exothermic reaction |
Water freezing, campfire burning, heater running |
|
|
Name examples of endothermic reaction |
Ice melting, egg being fried, water boiling |
|
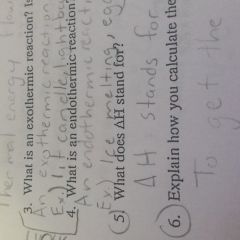
What does deltaH stand for |
It stands for the heat of the reaction |
|
|
Explain how you calculate the change in enthalpy for a given chemical reaction |
products-reactants |
|
|
What is the specific heat formula |
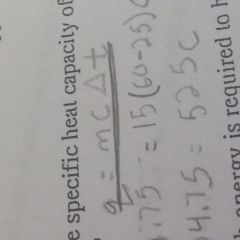
|
|
|
What do the symbols mean in the specific heat formula |
Q equals heat (J/cal) M equals Mass C equals specific heat T equals change in temperature |
|
|
Know how to label a phase diagram |
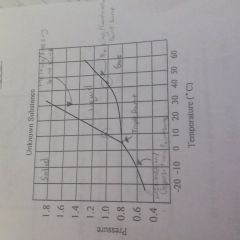
|
|
|
How do you find the normal boiling point |
Go at 1 atm and find the boiling point line |
|
|
How do you find normal melting point |
Go at 1 atm and find the melting point line |
|
|
What is the order of the elements in the phase diagram |
Solid liquid gas |
|
|
What is the difference between sublimation and deposition? |
Sublimation is solid to gas and deposition is gas to solid |
|
|
Know how to label a heating and cooling curve diagram |
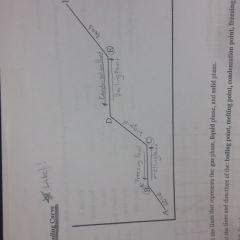
|
|
|
Know how to make reaction energy diagram |
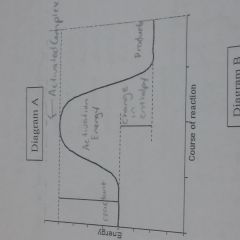
|
|
|
How do you get the change in enthalpy |
Products - reactants |
|
|
How do you get the activation energy |
Activated Complex - reactant |
|
|
How do you know if it's endothermic or exothermic |
Change in enthalpy or heat of reaction is negative then it is exothermic if it's positive then it is endothermic |
|
|
How do you do the extra-credit number 10 |
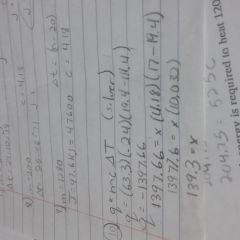
First step is to find the the heat of the reaction the mass of the object put in the water. Use the specific heat formula. Then insert that heat of the reaction into another specific heat formula but now use the waters specific heat and change in temperature. Solve to find the mass which is X and there's your answer. |

