![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
86 Cards in this Set
- Front
- Back
|
Metabolism |
Totality of an organism chemical reactions An emergent property of life from orderly interactions Transforming matter and energy |
|
|
Metabolic pathway |
Begins with a specific molecule and ends with a product Each step is catalyze by a specific enzyme |
|
|
Catabolic pathways |
Release energy by breaking down a more complex molecule to a simpler molecule Use that energy to do some other work ie: Cellulose respiration-breakdown of glucose into oxygen |
|
|
Anabolic pathways |
Energy consuming to build complex molecules from a simpler one ie: proteins built from amino acids |
|
|
Bioenergetics |
Study of how energy flows through living organisms |
|
|
Energy |
Capacity to cause change Can be converted from one form to another |
|
|
2 forms of energy |
Kinetic Potential |
|
|
Kinetic energy |
Associated with movement Thermal energy heat |
|
|
Thermal energy |
A form of kinetic energy Associated with random movement of atoms and molecules |
|
|
Heat |
The transfer of thermal energy to another object |
|
|
Potential energy |
Energy that matter possesses bc of its location or structure Chemical energy |
|
|
Chemical energy |
Potential energy available for release from a chemical reaction |
|

Front (Term)example |
Person on diving board has greater potential energy because he has more space to fall (his position vs people in the water). Diving converts potential energy to kinetic energy (moving molecules) Climbing ladder to build potential energy using kinetic energy Where did the energy go? |
|
|
Law of energy transformation |
Isolated system: unable to exchange energy or matter with its surroundings (water in thermos) Open system: energy and matter can be transferred btw the system and its surroundings |
|
|
Thermodynamics |
Study of energy transformations 2 laws: principle of conservation of energy Entropy and energy |
|
|
Principle of conservation of energy |

Energy of the universe is constant Energy can be transferred and transformed but cannot be created or destroyed |
|
|
Second law of thermodynamics |

Every energy transfer or transformation increases the entropy of the universe During energy transfer or transformation, some energy is unusable, often lost as heat. It creates more disorder in its surroundings: bear gives off heat and CO2. |
|
|
Entropy |
Chaos, Molecular disorder, Randomness The more random a collection of matter is, the greater the entropy Must be paid in order to transfer or transform energy |
|
|
Spontaneous processes |
Occur Without energy input, happen slow or quick Must increase the entropy of the universe “Energetically favorable” |
|
|
Nonspontaneous |
Processes that decrease entropy, will occur on if energy is provided |
|
|
Biological order and disorder |
Organisms create ordered structures from less organized forms of energy Replace ordered forms of natter and energy |
|
|
Free energy (G) |
Portion of a systems energy that is available to do work |
|
|
Free energy (G) |
Portion of a systems energy that is available to do work |
|
|
Change in free energy-delta (🔺) G |
Change is related to the change in enthalpy, change in entropy, and temp in Kelvin units |
|
|
Enthalpy |
Change in total energy Represented by delta H |
|
|
Formula for change in free energy |

Back (Definition) When delta G is a negative number, it is a spontaneous process. If not negative, then not spontaneous Spontaneous process that have free energy to do work, we can harness |
|
|
Equilibrium and open system |
Cells are not in equilibrium, constant flow of materials |
|
|
Negative delta G |
1. Drop in energy 2. If entropy is a larger number 3. If initial state is a greater number |
|
|
The lower the G... |
The more stable the system Less likely to change |
|
|
Measure of a systems instability... |
Free energy measures a systems tendency to change to a stable state or equilibrium During spontaneous change, free energy decreases and stability increases |
|
|
Exergonic reaction |
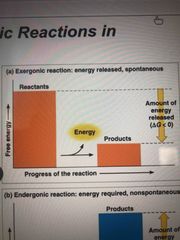
Proceeds with a net release of free energy Spontaneous Free energy exits -delta G |
|
|
Endergonic reaction |
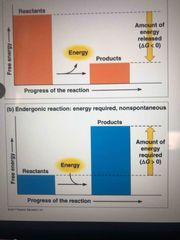
Absorbs free energy from its surroundings Nonspontaneous |
|
|
Magnitude of delta G |

The amount of energy required or released to do other work Same for forward or reverse reaction See example of glucose breakdown |
|
|
Forward and reverse of delta change |

Glucose production and breakdown: delta G= -686 kcal/mol Reverse of glucose via photosynthesis: delta G= +686 kcal/mol |
|
|
Equilibrium and closed system |
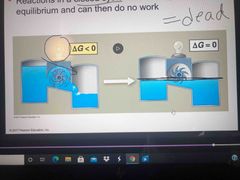
Closed systems eventually meet equilibrium and then no longer work A catabolic pathway in a cell releases free energy in a series of reactions |
|
|
Equilibrium and open system |
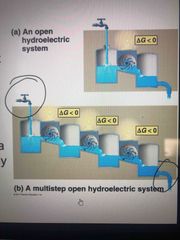
Cells are not in equilibrium, constant flow of materials |
|
|
Three types of work of cells |
Chemical Transport Mechanical |
|
|
Phosphorylated intermediate |
Phosphorylation: adding a phosphate group Endergonic reaction: Transferring a phosphate group to some other molecule, that recipient molecule is PI |
|
|
Regulation of enzymes |
A cell regulates metabolic pathways by inhibiting or activating enzymes |
|
|
Allosteric |
binds (not at active site) but makes the shape of enzymes inactive which changes shape of protein |
|
|
Competitive inhibitors |
Bonds at active site of enzyme Compete with substrate |
|
|
ATP |
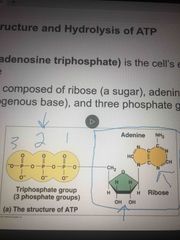
3 phosphate groups Energy currency. A monomer Contributes to making RNA miles clues |
|
|
Chemical work of cell |
Pushing endergonic reactions |
|
|
Transport work and ATP |
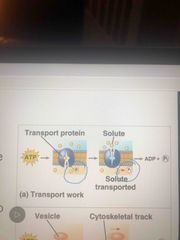
ATP hydrolysis does tranport and mechanical work Can change in protein shape and binding ability |
|
|
Mechanical work |
Such as contraction of muscle cells |
|
|
How does ATP produce energy? |
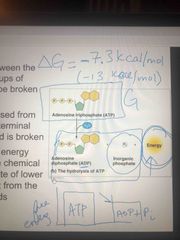
ATP is unstable Hydrolysis occurs breaking the bond of on phosphate group Then becomes ADP Breaking of bond creates an inorganic phosphate and then energy and makes it stable This process is delta G= -7.3kcal/mol (negative=exergonic) |
|
|
ATP |
3 phosphate groups Energy currency. A monomer Contributes to making RNA miles clues |
|
|
Regulation of enzymes |
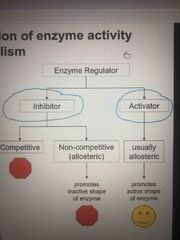
A cell regulates metabolic pathways by inhibiting or activating enzymes |
|
|
Transport work and ATP |
ATP hydrolysis does tranport and mechanical work Can change in protein shape and binding ability |
|
|
Competitive inhibitors |
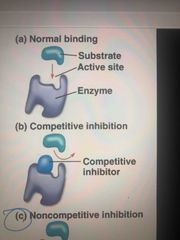
Bonds at active site of enzyme Compete with substrate |
|
|
Noncompetitive inhibitors |
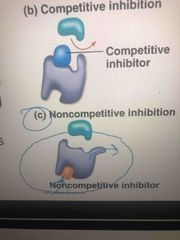
Bond to another part of an enzyme to change its shape and making the active site less effective Ex: toxins, poisons, pesticides, antibiotics |
|
|
Toxins and poisons as inhibitors |
Some toxins and poisons are not reversible so they may stay attached to the enzyme making it useless |
|
|
Allosteric regulation |
May either inhibit or stimulate an enzymes activity Occurs when it binds to a protein at one site and affects the protein function at another site |
|
|
How allosteric activation works |
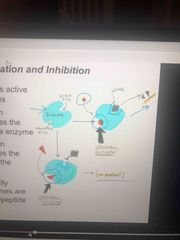
Each enzyme has active and inactive (regulatory site) forms The binding of an activator stabilizes the active form The binding of an inhibitor stabilizes the inactive form |
|
|
Activation energy (EA) |
Every chemical reaction btw molecules involved breaking and bond forming The initial energy needed to start chemical reactions is the free energy of activation or activation energy EA G |
|
|
It’s not just on or off..can have gray |
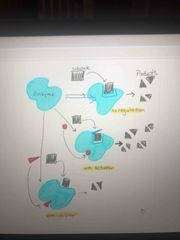
Enzyme without regulation, with activator, and with inhibitor show less product |
|
|
Cooperativity |
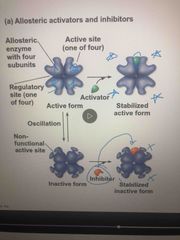
A form of allosteric regulation that can amplify enzyme activity When activator or inhibitor binds, it affects all 4 sites This is how hemoglobin binds-it increases more substances to bind |
|
|
Feedback inhibition |
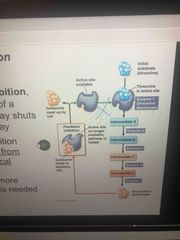
The end product of a metabolic pathway shuts down the pathway Prevents a cell from wasting chemical resources by synthesizing more product than is needed. |
|
|
Active site |
Region on the enzyme where the substrate binds |
|
|
Induced fit |
Chemical groups of the active site move into positions that enhance their ability to catalyze the reaction Hug it |
|
|
Enzyme cycle |
Take in reactant, hug it, lower activation energy, turn into products, release and ready to do it again |
|
|
Enzyme |
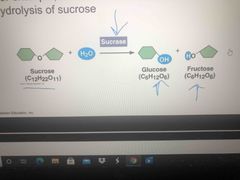
A catalytic protein Anything that ends in “ase” is an enzyme Enzymes do not make a spontaneous reaction nonspontaneous and they do not change delta G In the pic, if you add sucrose, it speeds the reaction up. |
|
|
Reverse and forward reactions of enzymes |
Go both forward and reverse Attempt to reach equilibrium so depends on concentration of products and reactants |
|
|
Activation energy (EA) |
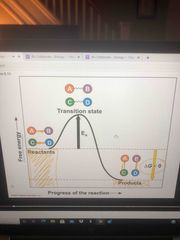
Every chemical reaction btw molecules involved breaking and bond forming The initial energy needed to start chemical reactions is the free energy of activation or activation energy EA G |
|
|
How Enzymes speed up reactions |
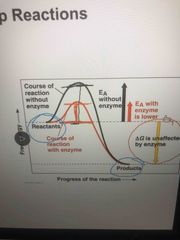
They lower the EA barrier (like lowering a fence so it is easier to jump over) Enzymes do not affect the change in free energy |
|
|
Temp and pH of enzyme |
Each enzyme has an optimal temp and pH in which it can function Optimal conditions favor the most active shapes for the enzyme molecule ie: typical human enzyme in pic is optimal at 37 Celsius. Still active at other temps up until about 50 Celsius but not as active as it is at 37 Same goes for pH |
|
|
Cofactors |
Nonprotein enzyme helper Inorganic (metals like iron) or organic(coenzyme which includes vitamins) |
|
|
Most allosterically regulated enzymes are made from... |
Polypeptide subunits |
|
|
Induced fit |
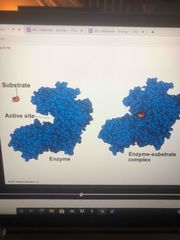
Chemical groups of the active site move into positions that enhance their ability to catalyze the reaction Hug it |
|
|
Enzyme cycle |
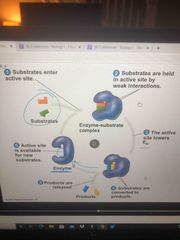
Take in reactant, hug it, lower activation energy, turn into products, release and ready to do it again |
|
|
Feedback inhibition |
The end product of a metabolic pathway shuts down the pathway Prevents a cell from wasting chemical resources by synthesizing more product than is needed. |
|
|
Reverse and forward reactions of enzymes |
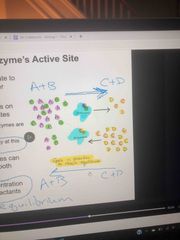
Go both forward and reverse Attempt to reach equilibrium so depends on concentration of products and reactants |
|
|
Evolution of enzymes |
Enzymes are proteins coded by genes (DNA) Mutations in genes can lead to changes in the amino acid which can change structure and function of the protein/enzyme This could be favored and thus evolution begins |
|
|
Temp and pH of enzyme |
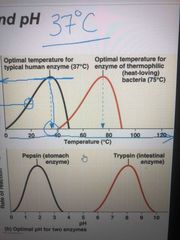
Each enzyme has an optimal temp and pH in which it can function Optimal conditions favor the most active shapes for the enzyme molecule ie: typical human enzyme in pic is optimal at 37 Celsius. Still active at other temps up until about 50 Celsius but not as active as it is at 37 Same goes for pH |
|
|
Enzyme |
A catalytic protein Anything that ends in “ase” is an enzyme Enzymes do not make a spontaneous reaction nonspontaneous and they do not change delta G In the pic, if you add sucrose, it speeds the reaction up. |
|
|
Bonds btw the phosphate groups of ATP are unstable because |
Negatively charged and repel one another And the terminal phosphate group is more stable in water than it is in ATP |
|
|
Allosteric proteins.. |
Exist in active and inactive forms Acted on by inhibitors Sensitive to environmental conditions |
|
|
The hydrolysis of ATP drives cellular work by |
Releasing free energy that can be coupled to other reactions |
|
|
The speed of the reaction is determined by |
The activation barrier of the reaction and the temperature (which determine how many reactants have energy to overcome the barrier) |
|
|
How do enzymes lower activation energy |
By locally concentrating the reactants |
|
|
Enzymes lower the activation energy of reactions but cannot change |
The free energy of products |
|
|
To overcome the activation energy barrier, an enzyme needs |
Heat Usually supplied from the environment |
|
|
What environmental conditions affect the rate of enzymes |
Substrate concentration pH Cooling and heating the enzyme |
|
|
How does pH disrupt enzyme activity |
High or low pH disrupts hydrogen bonds or ionic interactions thus change the shape |
|
|
Competitive inhibitors that bind covalently would be irreversible and those that bind weakly would be |
Reversible |
|
|
Stabilizing the structure of an enzyme in its active form |
Allosteric activation Remember regulatory form |

