![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
88 Cards in this Set
- Front
- Back
|
How are excess amino acids broken down by the body? |
They are separated into a nitrogen group, which is excreted via urea, and a carbon skeleton, which is used for energy production |
|
|
Which organ is the nitrogen group from the breakdown of amino acids transported to? |
The liver, where it is converted to urea for excretion via the kidneys |
|
|
What happens to the carbon skeleton produced by amino acid breakdown? |
It is fed into the TCA cycle at various points |
|
|
What are 'inborn errors of metabolism'? |
Errors in amino acid metabolism |
|
|
How much protein is broken down by our bodies per day? |
~300g |
|
|
How much protein should we consume in a day? How much is excreted per day? |
~100g for both, as a balance is required |
|
|
Where do we store proteins for use as an energy supply? |
We don't, any excess amino acids are immediately used as a form of energy |
|
|
What are the two sources of amino acids in our body? |
Dietary metabolism Protein breakdown |
|
|
How can amino acids be classified according to availability? |
Essential, non-essential, conditionally essential |
|
|
What is a 'conditionally essential' amino acid? |
One that we can produce, but not necessarily in sufficient quantities |
|
|
Describe histidine (His/H) according to all 3 classifications |
Essential, polar basic, glucogenic |
|
|
Describe isoleucine (Ile/I) according to all 3 classifications |
Essential, non-polar alkyl, gluco/ketogenic |
|
|
Describe leucine (Leu/L) according to all 3 classifications |
Essential, non-polar alkyl, ketogenic |
|
|
Describe lysine (Lys/K) according to all 3 classifications |
Essential, polar basic, ketogenic |
|
|
Describe methionine (Met/M) according to all 3 classifications |
Essential, non-polar alkyl, glucogenic |
|
|
Describe phenylalanine (Phe/F) according to all 3 classifications |
Essential, non-polar aromatic, gluco/ketogenic |
|
|
Describe threonine (Thr/T) according to all 3 classifications |
Essential, polar neutral, glucogenic |
|
|
Describe tryptophan (Trp/W) according to all 3 classifications |
Essential, non-polar aromatic, gluco/ketogenic |
|
|
Describe valine (Val/V) according to all 3 classifications |
Essential, non-polar alkyl, glucogenic |
|
|
Describe arginine (Arg/R) according to all 3 classifications |
Semi-essential, polar basic, glucogenic |
|
|
Describe cysteine (Cys/C) according to all 3 classifications |
Semi-essential, polar neutral, glucogenic |
|
|
Describe glutamine (Gln/Q) according to all 3 classifications |
Semi-essential, polar neutral, glucogenic |
|
|
Describe glycine (Gly/G) according to all 3 classifications |
Semi-essential, non-polar alkyl, glucogenic |
|
|
Describe proline (Pro/P) according to all 3 classifications |
Semi-essential, non-polar alkyl, glucogenic |
|
|
Describe serine (Ser/S) according to all 3 classifications |
Semi-essential, polar neutral, glucogenic |
|
|
Describe tyrosine (Tyr/Y) according to all 3 classifications |
Semi-essential, polar neutral, gluco/ketogenic |
|
|
Describe alanine (Ala/A) according to all 3 classifications |
Non-essential, non-polar alkyl, glucogenic |
|
|
Describe asparagine (Asn/N) according to all 3 classifications |
Non-essential, polar neutral, glucogenic |
|
|
Describe aspartic acid (Asp/D) according to all 3 classifications |
Non-essential, polar acidic, glucogenic |
|
|
Describe glutamic acid (Glu/E) according to all 3 classifications |
Non-essential, polar acidic, glucogenic |
|
|
Which class of enzymes breaks down dietary protein into amino acids and oligopeptides? Give two examples |
Proteolytic enzymes - pepsin and pancreatic enzymes |
|
|
How are oligopeptides further broken down into tri/dipeptides? |
By aminopeptidases from their amino-terminal end |
|
|
How are tri/dipeptides broken down into amino acids |
By peptidases |
|
|
How do many amino acid transporters work? |

They co-transport Na+ along with the amino acid, and rely on Na/K-ATPase to restore extracellular Na+ |
|
|
What might cause a protein to be degraded? |
- If it is damaged or incorrectly folded - if its production has been downregulated due to transcriptional activity - if it acts as a signalling protein that needs to be removed |
|
|
Where does most protein turnover occur? |
Skeletal muscle (41%) followed by liver (25%) |
|
|
How are proteins labelled for breakdown? Which organelle breaks the protein down? |
They are tagged with ubiquitin and broken down by the proteosome |
|
|
Describe the structure of the proteosome |
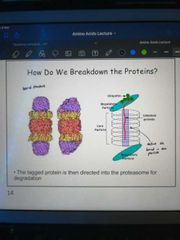
A barrel structure with its active site buried in the middle to prevent accidental breakdown of amino acids. It is also surrounded by regulatory particles to ensure only ubiquitinited proteins can get inside it |
|
|
Which enzyme tags proteins with ubiquitin? Describe how it works |
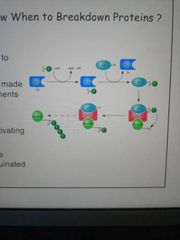
- Ubiquitin ligase has 3 subunits, E1/2/3 - E1 carries the ubiquitin and E2 activates it - E3 recognises the protein to be ubiquinated and binds to it, allowing E1 and E2 to pass the ubiquitin onto it |
|
|
What is Angelman's syndrome? |
A genetic disease caused by mutations in ubiquitin ligase, leading to severe motor and intellectual disability |
|
|
Is insulin a catabolic or anabolic hormone? |
Anabolic |
|
|
Are thyroid hormones/glucocorticoids catabolic or anabolic hormones? |
Catabolic |
|
|
How can amino acids be classified according to their side chain? |
Either polar or non-polar Polar can be further divided into acidic, neutral or basic Non-polar can be divided into alkyl or aromatic |
|
|
What is deamination? What are the 3 types? |
The removal of a nitrogen group from amino acids. The three types are oxidative, non-oxidative and hydrolytic |
|
|
Which is the only amino acid that undergoes rapid oxidative deamination? Describe the reaction |
Glutamate + NAD(P)+ => @-ketoglutarate + NAD(P)H + H+ + NH4+
Catalysed by glutamate dehydrogenase |
|
|
Describe glutamate dehydrogenase, including its location, coenzymes and allosteric regulation |
- Mostly expressed in liver/kidney - Can use either NAD+ or NADPH as a coenzyme - NAD+ used in oxidative deamination - NADPH used in reductive amination - deamination stimulated by ADP/GDP (low energy signals) - amination stimulated by ATP/GTP (high energy signals) |
|
|
If the amino acid is not glutamate, but needs to undergo oxidative deamination, what happens to it? |

It is transaminated, transferring its amino group to @-ketoglutarate, making glutamate and the amino acids corresponding a-ketoacid. The glutamate can then undergo deamination to remove the NH3 group |
|
|
Write the transamination reaction for alanine |
Alanine + @KG => glutamate + pyruvate (pyruvate is the @ketoacid for alanine) Catalysed by alanine transaminase |
|
|
Write the transamination reaction for aspartic acid |
Aspartic acid + @KG => glutamate + OAA (OAA is the @-ketoacid for aspartic acid) Catalysed by aspartate transaminase |
|
|
What do all transaminases require as a cofactor? |
Pyroxidal phosphate |
|
|
What is the ketoacid of alanine? |
Pyruvate |
|
|
What is the ketoacid of aspartate? |
OAA |
|
|
What is the ketoacid of leucine? |
2-oxo-3-methyl valerate |
|
|
What is the ketoacid of isoleucine? |
2-oxo-4-methyl valerate |
|
|
What is the ketoacid of valine? |
2-oxo-3-methyl butyrate |
|
|
Which two amino acids undergo non-oxidative deamination and why? Which enzymes carry this out? |
Serine and threonine because of the OH in their side chain. Serine/threonine dehydratase |
|
|
Which amino acids undergo hydrolytic deamination? |
Glutamine and asparagine |
|
|
Write out the reaction for the hydrolytic deamination of glutamine (the one for asparagine will be the same) |
Glutamine + H2O => glutamate + NH4+ Catalysed by glutaminase (asparaginase for asparagine) |
|
|
Describe the different locations where glutaminase is expressed and why |
Liver to generate urea Kidneys to generate NH4+ for acid base balance Neurons to assist in neurotransmission |
|
|
Write out the reverse reaction of the hydrolytic deamination of glutamine. Where does this process take place? |
Glutamate + NH4+ + ATP => Glutamine + ADP + Pi
Takes place in muscle via glutamate synthase |
|
|
Describe the urea cycle |
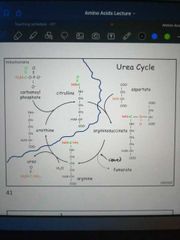
- Carbamoyl phosphate + ornithine => citrulline - citrulline + aspartate => argininosuccinate - argininosuccinate => fumarate + arginine Arginine + H2O => urea + ornithine |
|
|
Write out the equation for Carbamoyl phosphate synthesis |
HCO3- + NH4+ + 2ATP => Carbamoyl phosphate + 2ADP + 2Pi Catalysed by Carbamoyl phosphate synthetase 1 |
|
|
How is Carbamoyl phosphate synthetase 1 allosterically regulated? |
Activated by N-acetylglutamate |
|
|
What can cause a blockage of Carbamoyl phosphate synthesis and what does it lead to? |
Genetic defects in Carbamoyl phosphate synthetase 1, leads to hyperammonaemia |
|
|
What are glucogenic amino acids? |
Amino acids which are fed into the TCA cycle upon breakdown, they have side chains 3 carbons long or greater |
|
|
What are ketogenic amino acids? |
Amino acids with side chains of 2 carbons, which can be used to form Ketone bodies |
|
|
How can amino acids be classified according to their metabolic fate? |
Glucogenic Ketogenic Both |
|
|
Which 7 molecules are end-products of amino acid C-skeleton breakdown? Which are glucogenic and which are ketogenic? |
Pyruvate Fumarate OAA succinyl-coA @-ketoglutarate are glucogenic AcCoA Acetoacetyl-coA are ketogenic |
|
|
Which amino acids breakdown into pyruvate? |
Tryptophan Alanine Serine Glycine Cysteine Threonine |
|
|
Describe breakdown of deaminated tryptophan into pyruvate |
- Tryptophan => alanine + AcAcCoA - Alanine + @KG => Glutamate + pyruvate (alanine transaminase) |
|
|
Describe breakdown of deaminated alanine to pyruvate |
Alanine + @KG => glutamate + pyruvate (alanine transaminase) |
|
|
Describe the breakdown of deaminated glycine to pyruvate |
- Glycine + methylene-tetrahydrofolate => serine + tetrahydrofolate (serine hydroxymethyltransferase) - serine => Pyruvate + H2O + NH4 (serine dehydratase) |
|
|
Describe the breakdown of deaminated serine to pyruvate |
Serine => Pyruvate + H2O + NH4 (serine dehydratase) |
|
|
Describe the breakdown of deaminated cysteine to pyruvate |
Cysteine + H2O => Pyruvate + NH4 + thiocysteine (cystathionase) |
|
|
Describe the breakdown of deaminated phenylalanine to fumarate |
- Phenylalanine + tetrahydro-biopterin + O2 => Tyrosine + dihydro-biopterin + H2O (phenylalanine hydroxylase) - tyrosine => fumarate (tyrosine amino transferase) |
|
|
Phenylketonuria is caused by a deficiency in which enzyme? |
Phenylalanine hydroxylase |
|
|
Describe the breakdown of deaminated asparagine to OAA |
- asparagine + H2O => Aspartate + NH3 (asparaginase) - aspartate + @KG => OAA + glutamate (aminotransferase) |
|
|
Describe the breakdown of deaminated leucine to acetoacetate + AcCoA |
- Leucine => @ketoisocaproic acid (BCAA aminotransferase) - @ketoisocaproic acid => isovaleryl-coA (BC@ketoacid dehydrogenase) - isovaleryl-coA => Acetoacetate + AcCoA (dehydrogenation) |
|
|
Describe the breakdown of deaminated valine to succinyl-coA |
- Valine => @ketoisovaleric acid (BCAA aminotransferase) - @ketoisovaleric acid => isobutyryl-coA (BC@ketoacid dehydrogenase) - isobutyryl coA => propionyl-coA (dehydrogenation) - propionyl-coA => methylmalonyl-coA (biotin) - methylmalonyl-coA => succinyl-coA (5'deoxyadenosyl cobalamin) |
|
|
Describe the breakdown of deaminated isoleucine to acetyl-coA and succinyl-coA |
- Isoleucine => @keto-beta-methyl valeric acid (BCAA aminotransferase) - @keto-beta-methyl valeric acid => @-methyl-butyryl coA (BC@ketoacid dehydrogenase) - @-methyl-butyryl coA => AcCoA + propionyl coA (dehydrogenation) - propionyl-coA => methylmalonyl-coA (biotin) - methylmalonyl-coA => succinyl-coA (5'deoxyadenosyl cobalamin) |
|
|
Describe the breakdown of deaminated threonine to pyruvate |
Threonine => Pyruvate + CO2 (threonine dehydrogenase) |
|
|
Describe the breakdown of deaminated threonine to succinyl-coA |
- Threonine => @-ketobutyrate + NH4 (threonine dehydratase) - @ketobutyrate => succinyl-coA |
|
|
Describe the breakdown of deaminated methionine to succinyl-coA |
- Methionine => homocysteine ( via S-adenosyl-methionine) - homocysteine => @ketobutyrate (homocysteine desulphydrase) - @ketobutyrate => succinyl-coA |
|
|
Describe the breakdown of deaminated arginine to @KG |
- Arginine => ornithine (arginase) - ornithine => @KG (via urea cycle) |
|
|
Describe the breakdown of deaminated proline to @KG |
- Proline => glutamate (oxidation) - glutamate => @KG (glutamate dehydrogenase) |
|
|
Describe the breakdown of deaminated histidine to @KG |
- Histidine => glutamate (via histidase) - glutamate => @KG (glutamate dehydrogenase) |
|
|
Describe the breakdown of deaminated glutamine to @KG |
- glutamine => glutamate (glutaminase) - glutamate => @KG (glutamate dehydrogenase) |
|
|
Draw a map of the Krebs cycle, showing the entry points for all 20 amino acids |
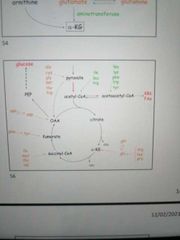
|

