![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
42 Cards in this Set
- Front
- Back
|
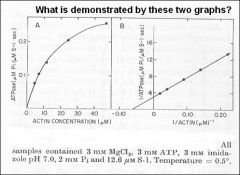
Minimum requirements for movement: Myosin + ATP + actin
Movement is the product of this reaction Need to have both substrates saturating (myosin and ATP) |
The cofactor, actin, behaves as a substrate.
Both ATP and actin follow Michaelis-Menten Kinetics |
|
|
Actin = Myosin + ATP <--> Actin < Myosin + ADP + Pi
|
“Movement” is one product of this reaction.
|
|
|
Use of a lever magnifies the conformational change and gives us movement power
Product of this enzymatic reaction is force |
What makes the conformational changes of myosin differ from other “non - motor ”enzymes?
|
|
|
1. Phosphorylation of myosin is required for smooth muscle contraction.
2. Phosphorylation of myosin enhances the contraction to a small extent in cardiac and skeletal muscle. 3. Myosin light chain kinase is activated by calcium-calmodulin. 4. Myosin phosphatase is also regulated. |
Muscle contraction is tightly regulated.
The regulation is tissue specific |
|
|
Myosin regulated/phosphorylated tissue
Acts as kinase. |
Smooth muscle contraction
Some cardiac and skeletal muscle. Phosphatase is also necessary and regulated |
|
|
________ is only present in striated muscle (good test question)
|
Troponin
|
|
|
1. Each tropomyosin lies along 7 actin protomers.
2. Tropomyosin position controls activity. 3. Both troponin and myosin can control the position of tropomyosin on actin. 4. Troponin is the major controller of tropomyosin position. 5. Troponin stabilizes the inactive position at low calcium concentrations |
Troponin-tropomyosin in striated muscle.
How actin regulates contraction |
|
|
What is the role of Ca2+ in striated (skeletal and cardiac) muscle contraction?
|
Complexity allows "allosteric" control that allows fine-tuned control of muscles
Troponin holds tropomyosin and actin in the unfavorable conformation till Ca2+ releases it |
|
|
Calcium causes troponin to let go of actin, allowing tropomyosin-troponin to move into a more active state.
What happens next? |
The troponin-actin interaction is lost when calcium binds. Tropomyosin can then move to a more active position.
|
|
|
Force producing crossbridges (FPB) further activate the system irrespective of calcium.
But such crossbridges are likely only during calcium activation |
ATP binding allows fully active FPB, Ca only makes FPB partially active
|
|
|
Inhibited + FPB -> Fully active
Partially active (+Ca2+) + FPB -> fully active |
Remember that Force producing crossbridges activate the system regardless of calcium.
However, most times will need calcium to prepare the crossbridging. |
|
|
If calcium is low, tropomyosin in _______ state
|
inactive
|
|
|
Calcium binds to ________
|
Troponin C (Tpn C)
|
|
|
Tropomyosin released to ___________ distribution…more active state.
|
equilibrium
|
|
|
If ATPase rate increases, what event will happen?
|
contraction occurs.
|
|
|
Myosin-ADP binding fully moves __________ to to active state.
|
tropomyosin
|
|
|
Nemaline Myopathy
|
skeletal muscle disorders
|
|
|
Familial Hypertrophic Cardiomyopathy
Dilated Cardiomyopathy Restrictive Cardiomyopathy |
cardiac muscle disorders
|
|
|
- Myopathies (acute quadriplegic)
- Cardiomyopathies (familial hypertrophic) - Hearing Loss (i.e. Usher syndrome) - Blindness (i.e. Usher syndrome) - Developmental irregularities - Pigment Loss (Griscelli syndrome) - Hematologic irregularities (thrombocytopenia; May-Hegglin anomaly) |
Myosin Motor Disorders:
not always what you expect… |
|
|
Any eukaryotic transport process is a target for disorders of molecular motors
|
As the volume grows the surface area cannot keep up.
|
|
|
- Myosin
- Actin or proteins that control polymerization - Regulatory proteins that control the activity of Myosin-Actin |
Muscle Disorders May result From Defects in…
|
|
|
Toxins like cytochalasin _______ polymerization.
|
inhibit
Actin polymerization highly regulated. |
|
|
Phalloidin _________ depolymerization.
|
prevents
Actin polymerization highly regulated. |
|
|
Contraction requires myosin, actin and accessory proteins.
Defects in any of these proteins = trouble Disorders resulting from myosin defects depend on the isozyme affected. Regulation of striated muscle is cooperative and requires troponin and tropomyosin. |
Calcium gives partial activation.
Full activation requires “activating” crossbridge binding. Alteration of the distribution among inactive, intermediate and active states may produce cardiomyopathies. |
|
|
The functional unit of a striated muscle is the sarcomere.
Shortening occurs when the thick (myosin) filaments slide past the thin (actin) filaments. Shortening requires ATP hydrolysis and an activating signal. Myosin binding to actin is essential for rapid ATP hydrolysis. Force is produced when the active site of myosin (S1) undergoes a large conformational change as ADP and Pi are released from the cleaved ATP |
Summary of movement
|
|
|
When ______ has bound ATP or ADP+Pi, the binding is weak and is very rapid.
This type of binding can occur in relaxed muscle. |
myosin
|
|
|
When Pi is released, myosin binds to actin differently. The binding is much stronger and the rate of detachment from actin is much slower.
This change in binding produces force. |
Actin is necessary both the rapid release of products and for movement.
|
|
|
The regulatory proteins of striated muscle are tropomyosin and troponin.
One troponin and one tropomyosin are bound to each ___ actin monomers. |
7
|
|
|
Troponin is not bound to actin in the presence of calcium and tropomyosin is free to move to its neutral position.
|
Here the ATPase rate is about 25% of the maximum value.
|
|
|
In the absence of calcium, troponin locks tropomyosin into the inactive position…very low ATPase activity.
|
No contraction can occur in this state.
|
|
|
High levels of myosin with bound ADP can trap the tropomyosin in the fully active state.
|
Not dependent on calcium
|
|
|
Some TnT mutations give more than 25% of the maximum rate in Calcium
|
Mutations of troponin change the normal distribution of states.
Lead to cardiomyopathies. |
|
|
Some mutations of TnI give less than 25% of the maximum rate in Calcium.
|
Mutations of troponin change the normal distribution of states.
Lead to cardiomyopathies. |
|
|
Thickening interferes with normal functioning of the heart by: - narrowing the outflow of the ventricle - reducing the ability of the heart to relax and fill with blood during the relaxation phase.
Reduces the ability of the valves of the heart to function properly. |
Hypertrophic Cardiomyopathy
Any situation that increases the contraction or rate of contraction of the heart muscle can worsen these symptoms. |
|
|
Prevalence of 1:500 individuals. Most common cause of sudden death in the young.
Cause of left ventricular hypertrophy is unexplained. Incompletely penetrant; different family members have different outcomes. Familial Cardiomyopathies result from mutations in any of the major contractile proteins. |
Hypertrophic Cardiomyopathy
|
|
|
Some mutations reduce ATPase activity and favor formation of the less active states.
Some mutations increase ATPase activity and favor formation of the more active states. |
These events lead to heart failure.
|
|
|
Regulation of _______ muscle is cooperative and requires troponin and tropomyosin.
Calcium gives partial activation. Full activation requires “activating” crossbridge binding. Alteration of the distribution among inactive, intermediate and active states may produce cardiomyopathies. |
striated
|
|
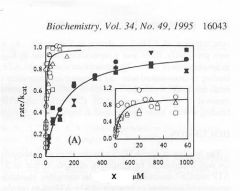
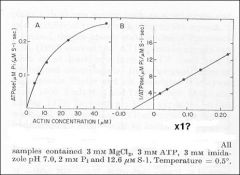
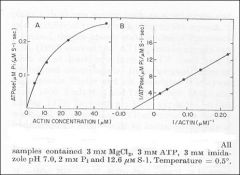
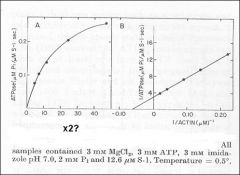
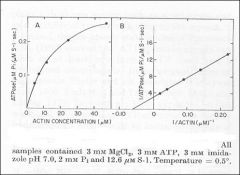
Identify X
|
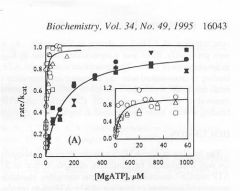
|
|
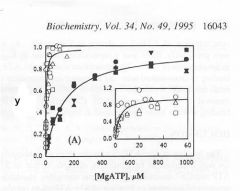
Identify Y
|
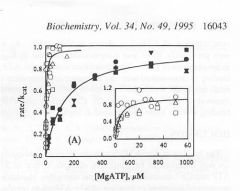
|
|
|
|
|
|
|
|
|
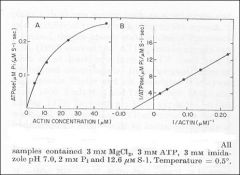
Dependence of actin ATPase on actin concentration
|

