![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
46 Cards in this Set
- Front
- Back
|
Atherosclerosis |
Disease that begins with the accumulation of lipids in the walls of large blood cells. These trapped lipids initiate inflammation by triggered the product of chemical signals |
|
|
Lipoproteins |
-Primary form of circulating lipid. - large in diameter(1000-5000) -protein content of 1-2% -primary function to transport dietary triaglycerols to adipose tissue and cholesterol to liver |
|
|
High density lipoproteins |
"Good cholesterol" -Primary function is to transport the body's excess cholesterol back to the liver for processing |
|
|
How are lipids obtained and synthesized |
By digesting food -they are synthesized from smaller precursors -they are used as a source of free energy -stored in adipose tissue |
|
|
Fatty acid oxidation (triaglycerols) |
Dietary triaglycerols are the primary source of fatty acids used as metabolic fuel |
|
|
Mobilization of fatty acids |
-Triaglycerols that are stored in adipose tissue are mobilized by an intracellular hormone sensitive lipase -the mobilized fatty acids travel through the blood stream, not as part of lipoproteins but bound to albumin |
|
|
What happens to fatty acids after they enter the cell |
They are either broken down or reesterfied to firm glycerol or other complex lipids |
|
|
Fatty acid degradation |
-To be oxidatively degraded, a fatty acid must first be activated -activation is a two step process catalyzed by acyl CoA synthetase -first fatty acid displaces the diphosphate group of ATP -then CoA displaces AMP to form acyl CoA -spontaneous and irreversible |
|
|
4 goals of lipid metabolism |
Fat storage Fatty acid oxidation Fatty acid biosynthesis Cholesterol |
|
|
Cholesterol from the diet to receptor mediate endocytosis |
Cholesteryl ester surrounded by ldl particle is attracted by ldl receptors in the cell, the ldl receptor brings the particle into the cell then disforms to allow the Cholesteryl ester to break down into amino acids and then cholesterol is formed |
|
|
Regulation of cholesterol biosynthesis |
1)intracellular [cholesterol] 2) hormonal systems 3) phosphate activity |
|
|
HMG-CoA reductase controls |
-energy levels -phosphorylation (unactive, AMP kinase)/dephosphorylation(active) -competitive inhibitors stains -genetic
- translation -SREBPs - translation - enzyme degradation - enzyme degradation |
|
|
Purpose of reducing nitrogen |
To make ammonia Get extra H+ from ferredoxin |
|
|
What is a "doxin" |
They are different forms of reduced cofactors, electron carriers, and nitrogenase |
|
|
Nitrogenase complex |
Electrons from reduced ferredoxin come in, ATP also comes in, changes reductase to nutrogenase which then converts nitrogen to ammonia |
|
|
PLP cofactor |
-This cofactor allows enzymes to have many different capabilities. -The benzene ring helps hide nitrogen ions and electrons because if the delocalized clouds -synthesizes amino acids |
|
|
Tetrahydrofolate (THF) |
-THF affects amino acids - handles carbons instead if nitrogen -adds and subtracts methyl groups is made easier with THF -provides many various oxidation states -helps make nucleic acids |
|
|
5 key intermediates |
Glutamate Glutamine-helps connect brain to bodily needs Aspartate Asparagine Alanine Some are from diet, others come from,key amino acids Typically regulated by cumulative feedback inhibition |
|
|
Glutamate/Glutamine shuttle |
Picks up 2 molecules of ammonium before they become toxic, both non MM |
|
|
Glutamate dehydrogenase |
Can create many reduced cofactors, but since it,works near equilibrium, it can also expell ammonium into tissue |
|
|
Glutamate synthase with regulation |
It's a neutral molecule that picks up 2 NH3 and delivers it to the liver for processing. Regulated by feedback inhibition and addition and removal of TAGs |
|
|
Cumulative feedback inhibition |
Since glutamine is a major regulatory point for many different molecules, they must act together in order to shut off the production of glutamine. But it's bad to have too much of one because it will then shut off the others that do not have enough |
|
|
Glutamine synthetase |
-Going from more active to less active by adding AMP. -Adding AMP decreases activity -adding AMP releases a PPi which can be broken down by phosphatase -addition of AMP tag can only knock out 1 particle in the total of 12, it only shuts off one active site -each AMP tag is analogous to phosphate to glycogen synthase. -multiple AMP can be added and each will affect the enzymes reducing activity. -adnyiltranferase is responsible for adding AMP -UMP tag us a signal to adnyiltransferase to not bind. But,instead to remove and keep the enzyme more actove |
|
|
NH3 balance and excretion |
-Urea cycle, if no need for nitrogen, push it through the nitrogen cycle in the liver and then dispose of them -the urea cycle is one cycle that we never want to stop. Goal is to make something toxic into something non toxic -abundance if either NH3 or aspartate will keep cycle moving |
|
|
The urea cycle |
-5 steps, start and ends in the mitochondria but intermediate stages are in the cytosol -levels of ATP is highest in the mitochondria so it makes sense to start the cycle in a place where energy is most abundant. |
|
|
5 reactions of urea cycle |
-Starts with carbamoyl phosphate, with the addition of 2 ATP, converts to citrulline -citrulline converts to arginino-succinate by adding ATP -arginino-succinate is converted to arginine -arginine concerted to ornithine, releasing urea Then back to the mitochondria |
|
|
Krebs bi-cycle |
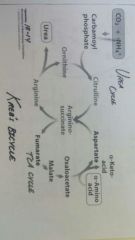
TCA cycle and urea cycle working together to continue to produce cofactors to keep urea cycle flowing |
|
|
Heme biosynthesis |
-Produces porphroine rings. -Major regulatory complex is porphobilinogen deaminase -without porphobilinogen deaminase, can't make porphirine rings -porphobilinogen deaminase releases 4 ammonia |
|
|
5-aminolevulinate synthase (ALAS) |
-iron must bind to transferran. Brings complex into cytosol then they are released -an iron pool is,formed. It is important because mRNA that makes the porphorine rings starts in the mitochondria -IREBP is a way to **** off certain products. No iron can recognize all mRNA and shut off transcription/translation |
|
|
Roles of nucleotides in biology |
-DNA/RNA bases -energy charge (metabolism) -physiology -adenine(sleep, blood flow, heart rhythm, vasodialation) -ADP -cAMP, cGMP (second messangers) -GDP/GTP -precursors/coenzyme -"Active" metabolites -allosteric regulators -ATCase (non-MM, key in pyrimidine synthase) |
|
|
Salvage pathway |
-generates pool of nucleic acids -activated ribise + base --> nucleotide -releases ammonium |
|
|
DeNovo pathway |
Activated ribose +amino acids+ ATP + CO2-->Nucleotide -synthetic |
|
|
PRPP synthetase |
-requires Pi for activity even though it uses ATP as Pi donor -activity is decreased by high [ADP] and 2,3-BPG -[PRPP] low in resting cells but increases during times of cell division -high G6P will increase the activity if PRPP synthetase |
|
|
PRPP reactions |
-Purine biosynthesis -Purine salvage -pyrimidine biosynthesis -Pyrimidine salvage -synthesis of NAD+ |
|
|
Regulation of Pyrimidine biosynthesis |
Regulation is at the beginning -ATCase turns it into nitrogenous base -regulation of CPSI without affecting CPSII in mitochondria -feedback inhibition |
|
|
Pyrimidine metabolism |

[CTP] increases, inhibits, non-MM, not allosteric affector. -dUMP is the salvage if Pyrimidine, must start with dUMP to reach dTTP. -without dTTP, proper DNA synthesis cannot occur. -activity goes down |
|
|
Purine products |
AMP inhibits its own pathway while activating the production of GMP and vice versa |
|
|
What is Gout |
It's when more urate molecules are in the tissue. More urate in tissues can cause arthritis |
|
|
What can problems with adenosine deamirase cause |
It can cause sorts if fetal auto immune disease. Probability if getting leukemia |
|
|
What is the importance of xanthylate oxidase |
It is important because you are taking a molecule and converting it to a uric product |
|
|
Ribonucleotide reductase |
Manages to make all nucleic acids in appropriate ratios and continue with DNA synthesis. -it's a global on off switch. Allosterically body guarded |
|
|
Monoclonal ABS |
-Antibody response -involves nucleic metabolism -helps recognize HIV and,other viruses by antibodies reacting with certain things in the blood. |
|
|
Glutamine synthetase cycle |
Since glutamine synthetase is an enzyme that plays a crucial role in metabolism of nitrogen by catalyzing the condensation of glutamate and ammonia, it needs to he regulated. -uridylyltransferase binds UTP to adenylyltransferase, which can then remove the phosphorylating group from glutamine synthetase and allow it to become more active and make glutamine |
|
|
Urea cycle (in depth) |
-CPSI combines ammonia with carbamoyl phosphate and or nothing to create citrulline that wil then move out to the cytosol -aspartame then will bind to citrulline producing argininosuccinate -fumarate is then released -arginine remains -urea is then taken from the clue and arginine is converted back to ornithine |
|
|
Importance of inasinate |
It's part of nucleotide metabolism even though it's not a DNA or RNA base. -important in fetal development |
|
|
Carbamoyl phosphate synthetase II (CPS II) |
Nh3 + hco3-+2ATP--> carbamoyl phosphate -Aids in Pyrimidine biosynthesis - created a Pyrimidine ring that prpp binds to to great UTP, which can then go to CTP(rna) |

