![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
75 Cards in this Set
- Front
- Back
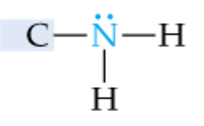
|
Primary Amine |
|
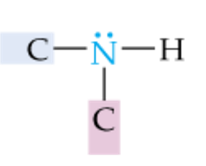
|
Secondary Amine |
|
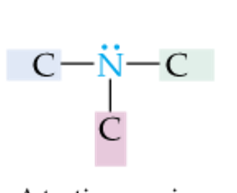
|
Tertiary Amine |
|
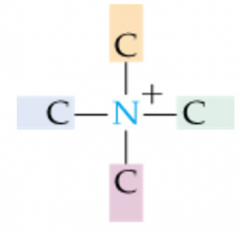
|
Quaternary Ammonium Ion |
|
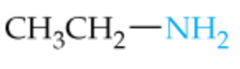
Name this Amine |
Ethylamine |
|
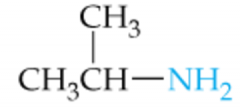
Name this Amine |
Isopropylamine |
|
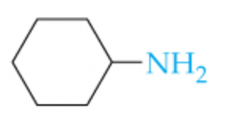
Name this Amine |
Cyclohexylamine |
|
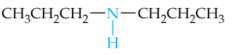
Name this Amine |
Dipropylamine |
|
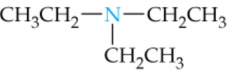
Name this Amine |
Triethylamine |
|
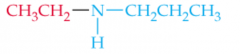
Name this Amine |
N-Ethylpropylamine |
|
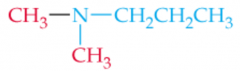
Name this Amine |
N,N-Dimethlypropylamine |
|
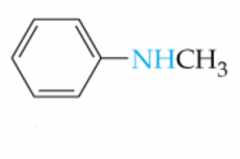
Name this Amine |
N-Methylaniline |
|
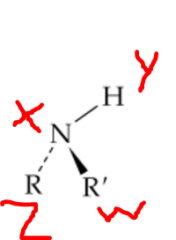
Where would you expect hydrogen bonding to occur? |
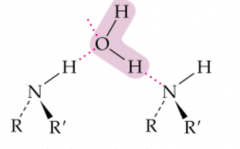
X and Y |
|
|
Which amines have higher boiling points? (primary, secondary, tertiary) |
Primary and Secondary, they can hydrogen bond with each other. |
|
|
Are Amines water soluble?
|
Yes, with up to 4 carbons
|
|
|
Do Amines have a smell?
|
Yes, they can smell like rotting meat, stale fish, and ammonia
|
|
|
Are Amines an acid or base? |
They are a weak base |
|

What would this reaction result in? |
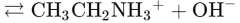
|
|
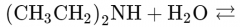
What would this reaction result in? |
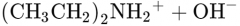
|
|
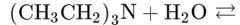
What would this reaction result in? |
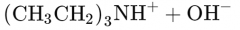
|
|
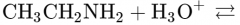
What would this reaction result in? |
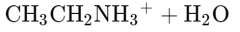
|
|

What would this reaction result in? |
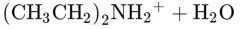
|
|
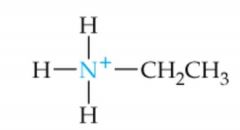
What is the name of this amine ion? |
Ethylammonium ion |
|
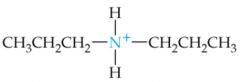
What is the name of this amine ion? |
Dipropylammonium ion |
|
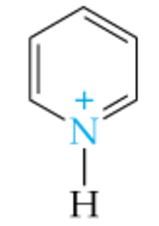
What is the name of this amine ion? |
Pyridinium ion |
|
|
Place in these in order from most basic to least basic: Ammonia, Nonaromatic amines, Aromatic Amines |
Nonaromatic Amines> Ammonia> Aromatic Amines |
|
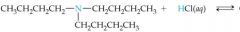
What would this reaction result in? |
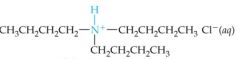
|
|
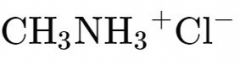
What is the name? |
Methylammonium Chloride |
|
|
Are ammonium salts water soluble? |
Yes, they are more water soluble than neutral amines. |
|
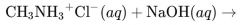
What would result from this reaction? |
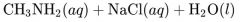
|
|
|
Properties of Quaternary Ammonium Salts |
Nitrogen has a permanent positive charge, neither acidic nor basic, structures are unaffected by changes in pH. |
|
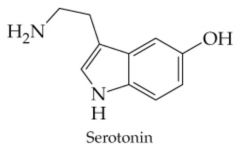
Does this compound contain an Alkaloid?
|
Yes, Alkaloids typically come from plants, are basic, and very bitter tasting. |
|
|
What is a Carbonyl group?
|
C double bonded to an O |
|
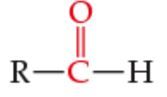
What is the functional group? |
Aldehyde |
|
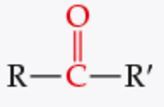
What is the functional group? |
Ketone |
|
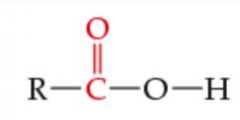
What is the functional group? |
Carboxylic Acid |
|
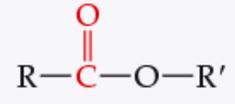
What is the functional group? |
Ester |
|
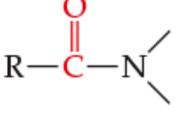
What is the functional Group? |
Amide |
|
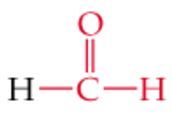
What is the name? |
Formaldehyde
|
|
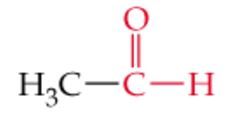
What is the name? |
Acetaldehyde |
|
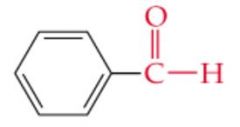
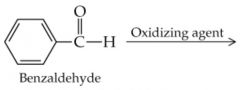
What is the name? |
Benzaldehyde |
|
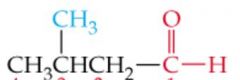
What is the name?
|
3-Methylbutanal |
|
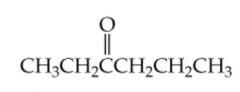
What is the name? |
3-Hexanone |
|
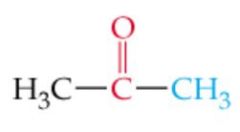
What is the name? |
Acetone |
|
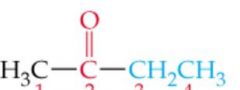
What is the name? |
Methyl Ethyl Ketone/ 2-Butanone |
|
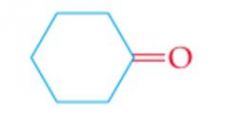
What is the name? |
Cyclohexanone |
|
|
Are Aldehydes and Ketones soluble in water? |
Yes, with fewer than four carbons they are. |
|
|
Put these in order from lowest to highest boiling point: Aldehydes, amines, carboxylic acid, ethers, hydrocarbons, 1°, 2° amines, diols. |
Carboxylic Acid> Diols> 1°,2°amines> aldehydes, Ketones> Ethers> hydrocarbons
|
|
|
Do aldehydes and ketones have pleasant smells/ tastes? |
Yes, many aromas and flavors come from them. |
|
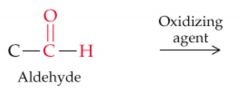
What results from this reaction? |
|
|
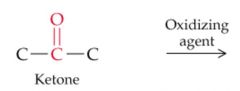
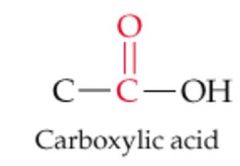
What results from this reaction? |
Nothing, No Reaction |
|
What results from this reaction? |
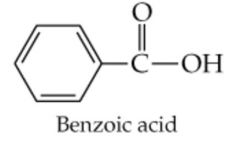
|
|
|
What will result in a positive Tollen's test? |
Aldehydes |
|
|
What will result in a positive Benedict's test? |
Aldehydes and Ketones with an -OH on the carbon next to the carbonyl |
|
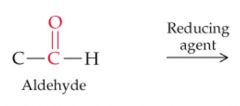
What will this reaction result in? |
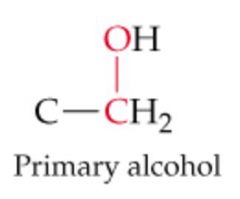
|
|
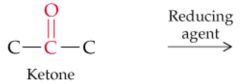
What will this reaction result in? |
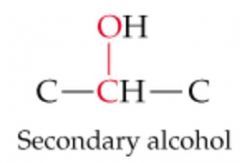
|
|
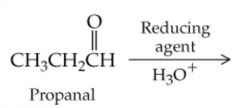
What will this reaction result in? |
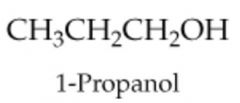
|
|
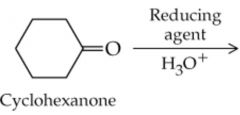
What will this reaction result in? |
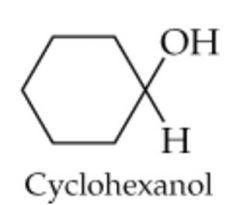
|
|
|
How are Hemiacetals formed? |
Through an addition reaction between an alcohol and an aldehyde or Ketone. |
|
|
How is an Acetal formed? |
When a hemiacetal and an alcohol go through a substitution reaction. |
|
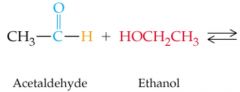
What results in this reaction?
|
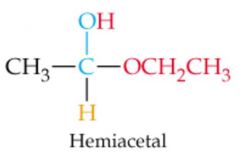
|
|
|
How can an Acetal be used to form a Hemiacetal? |
Through Hydrolysis with an acid catalyst |
|
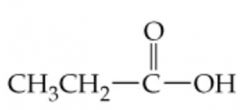
Name this |
Propanoic Acid |
|
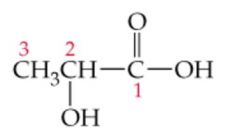
Name this |
Lactic Acid or 2-hydroxypropanoic acid |
|
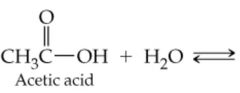
What would result in this reaction |
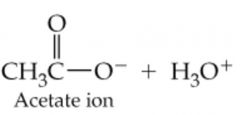
|
|
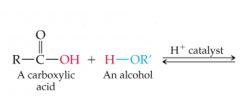
What results in this reaction? |
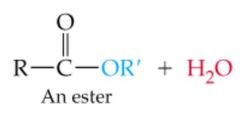
|
|
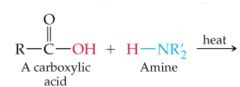
What results in this reaction |
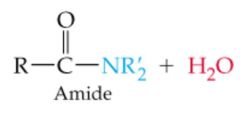
|
|
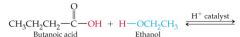
What results in this reaction |
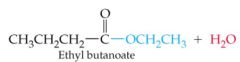
|
|
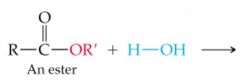
What results in this reaction |
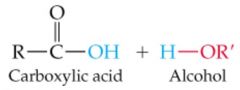
|
|
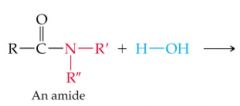
What results in this reaction |
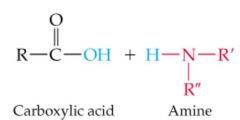
|
|
|
How do you produce Polyamides? |
By reacting diamines with diacids |
|
|
How do you produce Polyesters? |
By reacting diacids with dialcohols |
|
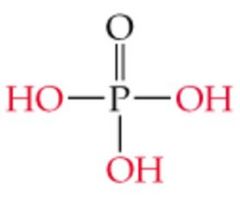
Name this |
Phosphoric Acid |
|
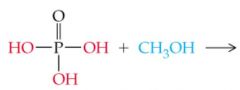
What does this reaction result in |
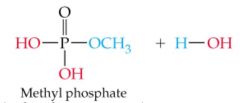
|
|
|
s |
s |

