![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
168 Cards in this Set
- Front
- Back
|
Chemical Evolution
|
Chemical compounds combine to form more complex ones.
|
|
|
Radiometric Dating
|
Finding how old something is by counting the half lives.
|
|
|
Isotopes
|
"Equal places" Forms of an element with different numbers of neutrons.
|
|
|
Mass Number
|
The sum of protons and neutrons of the most common isotope.
|
|
|
Atomic Number
|
The number of protons.
|
|
|
Orbitals
|
A region in which electrons move around. Each one contains two electrons.
|
|
|
Electron Shells
|
Groups of orbitals.
|
|
|
Valence Shell
|
The outermost shell.
|
|
|
Covalent Bond
|
Atoms are held together by shared electrons.
|
|
|
Nonpolar Covalent Bond
|
An equal sharing of electrons.
|
|
|
Polar Covalent Bonds
|
Asymmetric sharing of electrons.
|
|
|
Electronegativity
|
The tendency of some atoms to hold electrons more tightly.
|
|
|
O N C H
|
Highest electronegativity to lowest. Also, make up 96.3% of the human body.
|
|
|
Ion
|
A charged atom or molecule.
|
|
|
Ionic Bond
|
Electrons are transfered from one atom to another.
|
|
|
Cation
|
Positively charged ion.
|
|
|
Anion
|
Negatively charged ion.
|
|
|
Mole
|
6.022 x 10^23
|
|
|
Molarity
|
The number of moles of a substance per liter of a solution.
|
|
|
CH4
|
Methane
|
|
|
NH3
|
Ammonia
|
|
|
Chemical Reaction
|
A substance is combined with others or broken down into another substance.
|
|
|
Endothermic
|
Heating within. ie liquid water to steam.
|
|
|
Exothermic
|
Outside heating. ie water vapor to liquid.
|
|
|
Chemical Equilibrium
|
a dynamic but stable state in which the concentration of reactants remain constant.
|
|
|
Potential Energy
|
Stored energy
|
|
|
Kinetic Energy
|
The energy of motion.
|
|
|
Thermal Energy
|
The temperature. (The kinetic energy of molecular motion).
|
|
|
Gibbs Free-Energy Exchange
|
A measure of the change in potential energy and entropy that occurs in a chemical reaction. Determines if the reation will be spontaneous.
|
|
|
Exergonic
|
Spontaneous reactions that release energy and increase entropy.
|
|
|
Endergonic
|
Non spontaneous reations that store energy and require an input of energy.
|
|
|
Free Radical
|
Atom with upared electron that is unstable. ie single Hydrogen atom.
|
|
|
Chemical Energy
|
Potential energy stored in chemical bonds.
|
|
|
Redox
|
Reduction Oxidation reaction. Chemical reactions that involve the loss or gain of an election. OIL RIG (Oxidation Is Loss, Reduction Is Gain)
|
|
|
Organic Molecules
|
Molecules that contain carbon.
|
|
|
Hydrogen Bond
|
A weak bond that is formed from the partial positive charge of a hydrogen atom on one molecule with a partial negative charge of an atom (usually O or N) on another molecule.
|
|
|
Specific Heat
|
The amount of energy required to raise the temperature of 1 gram of a substance by 1 degree Celsius.
|
|
|
Water
|
Highly poloar covalent bonds between Oxygen and Hydrogen. An extraordinarily high specific heat. Its polarity makes it the universial solvent. Can act as both an acid and a base.
|
|
|
Heat of Vaporization
|
The energy required to change 1 gram from a liquid to a gas.
|
|
|
Acids
|
Substances that give up protons during chemical reations. (H+, hydrogen ion is simply a proton)
|
|
|
Bases
|
Molecules or ions that acquire protons during chemical reactions (OH-, hydroxide ion)
|
|
|
Acid-Base Reactions
|
Reactions that involve a transfer of protons.
|
|
|
pH scale
|
Power of Hydrogen, the measure of hydrogen ions H+. 14 is basic 1 is acidic. Pure water is 7 neutral.
|
|
|
Stanley Miller's 1953 experiment
|
Started with water methane (CH4) Ammonia (NH3) and Hydrogen (H2). Added heat and sparks. Results contained formaldehyde (H2CO), hydrogen cyanide (HCN) and amino acids.
|
|
|
Isomers
|
Molecules that have the some molecular formula but different structure. eg. sucrose and fructose
|
|
|
Monomer
|
"one-part" a single molecular subunit such as an amino acid or sugar.
|
|
|
Polymer
|
"many-parts" Many monomers bound together.
|
|
|
Polymerization
|
The process of monomers linking form polymers.
|
|
|
Macromolecule
|
Large molecule that is made up of smaller molecules joined together.
|
|
|
Protein
|
Linear macromolecule, a polymer made up of amino acid monomers.
|
|
|
Condensation Reactions
|
Or Dehydration Reactions, monomers polymerize through these. The bond results in the loss of a water molecule.
|
|
|
Hydrolysis
|
Breaking apart polymers by adding a water molecule.
|
|
|
Peptide Bond
|
Strong covalent C-N bond between two amino acid residues in a peptide or protein.
|
|
|
Polypeptide
|
A chain of amino acids linked by peptide bonds.
|
|
|
Oligopeptide
|
"few peptides" fewer than 50 amino acids linked together.
|
|
|
Hydrophobic
|
Scared of water, like Valine.
|
|
|
Hydrophilic
|
Loves water like Lysine
|
|
|
Amino Acid
|
Amino group linked to a carboxyl (acid) group and an H, C, and R.
|
|
|
Primary Structure
|
Unique sequence of amino acids that form protein's peptide-bonded backbone.
|
|
|
Secondary Structure
|
Shape of peptide-bonded backbone that is formed from hydrogen bonds that make an alpha-helix or a beta-pleated sheet.
|
|
|
Tertiary Structure
|
Define the 3-D shape of protein. Made up of alpha-helices and beta-pleated sheets.
|
|
|
Quaternary Structure
|
Shape produced by combinations of polypeptides.
|
|
|
Disulfide bonds
|
A covalet bond between two sulfur atoms, contributes to tertiary structure of protein.
|
|
|
Van Der Waals Interactions
|
Weak electrical attraction between two hydrophobic side chains. Contributes to tertiary structure.
|
|
|
Denatured
|
Unfolding a protein's stucture by breaking hydrogen and disulfide bonds.
|
|
|
Molecular Chaperone
|
Protiens that help fold newly synthesized protiens.
|
|
|
Lipids
|
Nonpolar hydrophobic organic compound. Types include: Steroids, Phospholipids and Fats.
|
|
|
Sulfhydryl
|
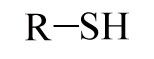
-
|
|
|
Hydroxyl
|
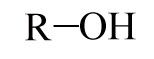
-
|
|
|
Carbonyl
|
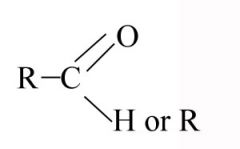
-
|
|
|
Carboxyl
|
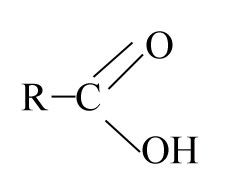
-
|
|
|
Phosphate
|
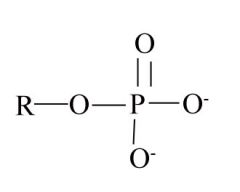
-
|
|
|
Amino
|
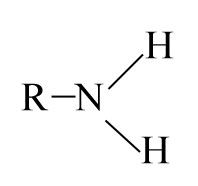
-
|
|
|
Peptide Bond
|
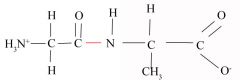
-
|
|
|
Non-Ionized Amino Acid
|
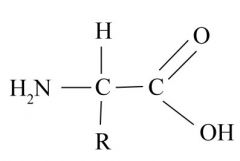
-
|
|
|
Ionized Amino Acid
|
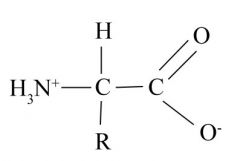
-
|
|
|
Fatty Acid
|
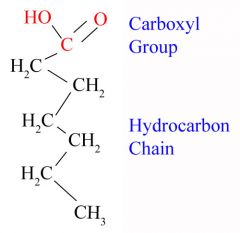
-
|
|
|
Steroid Image
|
page 107
|
|
|
Phospolipid Image
|
page 107
|
|
|
Fat Image
|
page 107
|
|
|
Ester Linkage
|
Covalent bond between fatty acid and glycerol to form fat or phospholipid.
|
|
|
Dehydration Reaction
|
Joins fatty acid to glycerol when water leaves.
|
|
|
Phospolipid Bilayer
|
Created when two sheets of lipid molecules align with their hydrophobic tails facing each other.
|
|
|
Amphipathic
|
Compounds that contain hydrophobic and hydrophilic elements.
|
|
|
Unsaturated
|
A carbon double bond exists in the hydrocarbon tail of a fatty acid and produces a kink. Kinks in a lipid bilayer allow for higer permiability.
|
|
|
Saturated
|
No carbon double bond exists in the hydrocarbon tail of a fatty acid. Lipid bilayers with these are less permiable than unsaturated.
|
|
|
Solutes
|
Dissolved molecules and ions.
|
|
|
Concentration gradient
|
Difference in concentration.
|
|
|
Diffusion
|
Movement of molecules and ions from high concentration to low concentration.
|
|
|
Osmosis
|
Diffusion of water, occurs with a selectively permiable membrane.
|
|
|
Isotonic Solution
|
The concentration of solutes is the same on both sides of a membrane
|
|
|
Hypotonic Solution
|
The conentration of a solute is less on the outside of a membrane so water enters and causes it to burst.
|
|
|
Hypertonic Solution
|
The concentration of a solvent is greater on the outside of a membrane so water leaves and causes it to shrivel.
|
|
|
Integral Membrane Proteins
|
Or transmembrane proteins have elements facing both interior and exterior surfaces of a membrane.
|
|
|
Peripheral Membrane Proteins
|
Found only on one side of a membrane.
|
|
|
Diffusion Membrane Transport
|
Passive movement along an electrochemical gradient through a membrane or channel.
|
|
|
Facilitated Diffusion Membrane Transport
|
A transmembrane transport protein moves the molecule without the expeniture of energy.
|
|
|
Active Membrane Transport
|
Transports ions against their electrochemical gradient. The energy required for this is from ATP converting to ADP.
|
|
|
Ionophores
|
"ion-mover" used in facilited diffusion.
|
|
|
Cytoplasm
|
"cell-formed" contents of the cell inside the membrane.
|
|
|
Oraganelle
|
"little organ" a membrane bound compartment in the cytoplasm that contains enzymes.
|
|
|
Cytosol
|
The fluid portion of the cytoplasm.
|
|
|
Lumen
|
The interior of any sac-like structure. (technically cytoplasm is a lumen).
|
|
|
Endocytosis
|
"inside-cell-act" any pinching off of the plasm a membrane that results in the uptake of material from outside the cell.
|
|
|
Exocytosis
|
The secretion of cellular contents to the outside of the cell by fusion of viscles to the plasma membrane.
|
|
|
Prokaryotes
|
No membrane-bound nucleus but nucleoid. Flagella for movement. Plasmids with DNA.
|
|
|
Eukaryotes
|
Membrane bound nucleus. Larger than prokaryotes.
|
|
|
Plant Cell
|
Has a cell wall in addition to plasma membrane. Has chloroplasts which are organelles that convert sunlight to energy via photosynthesis.
|
|
|
Endomembrane System
|
Golgi Apparatus, Lysosomes and ER. The primary center for protein and lipid synthesis.
|
|
|
Nuclear Lamina
|
Fibrous proteins that stiffen the envelope and helps organize chromasomes.
|
|
|
Nuclear Envelope
|
Double membrane that surrounds the nucleus.
|
|
|
Heterochromatin
|
Chromasomes that are highly compact and supercoiled.
|
|
|
Euchrmatin
|
Chromasomes that are unwound into long, filamentous strands.
|
|
|
Nucleolus
|
Manufactures rRNA molecules.
|
|
|
Ribosomes
|
Creates proteins
|
|
|
Rough ER
|
Membrane bound sacs and tubules containing ribsomes.
|
|
|
Smooth ER
|
ER without ribosomes that synthesis lipids. Also stores calcium.
|
|
|
Golgi Apparatus
|
Processes, sorts, and ships proteins from the ER. Made up of sacs called Cisternae.
|
|
|
Peroxisomes
|
Centers for oxidation reactions. Catalyses hdrogen peroxide H202. They grow and divide independently.
|
|
|
Lysosomes
|
Solid waste processing and storage. Called vacuoles in plant cells.
|
|
|
Phagocytosis
|
"eat-cell-act" the plasma membrane of a cell surrounds food and engulfs it. it then goes to the lysosome.
|
|
|
Autophagy
|
"samge-eating" damaged organelles are surrounded by a membrane and delivered to lysosome.
|
|
|
Receptor-Mediated Endocytosis
|
Food binds to membrande protiens that act as receptors. The plasma membrane folds and pinches off creating a vesicle called a early endosome. Digestive enzymes come from the golgi apparatus and mature to late endosomes and eventually lysosomes.
|
|
|
Pinocytosis
|
"drink cell-act" vesicles form on the plasma membrane the fluid is not transported to the lysosomes.
|
|
|
Mitochondria
|
Double-membraned made up of mitochondrial matrix. Produces ATP.
|
|
|
Chloroplasts
|
Site of photosynthesis. Double-membrane. Thlakoids are flattend vesicles. Granum are stacked thlakoids. Stroma other regions with enzymes.
|
|
|
Cell Wall
|
Only in plant cells. Contains Lignin for protection.
|
|
|
Nuclear Transportation
|
NLS is the package with zip code. Importins are the truck. ATP is the gas. Ran is the unloading crew. GDP/GTP is the supervisor.
|
|
|
Cytoskeleton
|
Made of actin filaments and microtubules that provide structural support.
|
|
|
Vesicle Transport Requires
|
ATP (Gas), microtubules (tracks), vesicle (cargo), kinesin train.
|
|
|
Kinesin and Dynein
|
Motor protiens
|
|
|
Flagellum
|
Function in movement. In eukaryotic cells they are made of microtubules. In bacteria they are made of a protein called flagellin.
|
|
|
How are Cilia and Flagella constructed?
|
The axoneme has a 9 + 2 microtubule arangement. And the basal body has a 9 + 0.
|
|
|
Pyrimidines
|
Single-ringed nitrogenous bases. cyosine, uricil (rna only), thymine (dna only)
|
|
|
Purines
|
Double-ringed nitrogenous bases. guanine and adenine.
|
|
|
What keeps genetic material together?
|
Hydrogen bond.
|
|
|
Ribozyme
|
An RNA molecule that acts as a catalyst.
|
|
|
Nucleotide
|
Consists of a phosphate group, 5-carbon sugar and a nitrogenous base.
|
|
|
Cellulose
|
The most abundant organic molecule. Makes up the cell wall in plant cells.
|
|
|
Lignin
|
Makes the secondary cell wall in plant cells. Makes wood hard.
|
|
|
Middle Lamina
|
The space between cell walls composed mostly of pectins.
|
|
|
Plasmodesmata
|
In plants, the gaps in cell walls that create conections between cytoplasms. Tubule of ER pass through these.
|
|
|
ECM
|
Extra cellular matrix. Made up of fibroconectins, integrins and collagen.
|
|
|
Selective Adhesion
|
When cells in tissue bond to the same type.
|
|
|
Desmosomes
|
Rivets that hold animal cells together by binding their cytoskeletons.
|
|
|
Gap Junctions
|
Span membranes in animal cells and admin small molecules such as amino acids, sugars and nucleotides.
|
|
|
Tight Junctions
|
Animal cell-cell attachment that form a quilt pattern and a water tight seal.
|
|
|
Ligand
|
Any molecule that binds to a receptor molecule.
|
|
|
Hormone
|
A ligand that acts as an intercellular signal.
|
|
|
Signal Receptor
|
Proteins that bind to a signal and change conformity or activity in response.
|
|
|
G Protien
|
Bind to GTP and GDP. Signal transducers that have a time-delayed turn off switch.
|
|
|
Receptor Tyrosine Kinases
|
Starts a phosphorylation cascade that uses ATP send signal other protiens.
|
|
|
Induced Fit
|
The change in shape of an enzyme in response to a reactant molecule binding to the active site.
|
|
|
Catalyst
|
A substance that lowers the activation energy of a reaction
|
|
|
Enzyme
|
Proteins that catalyze reactions.
|
|
|
Enzyme Cofactors
|
Atoms or molecules that are not part of the enzymes structure that are required for the enzyme to function.
|
|
|
Competitive Inhibition
|
Catalysis is inhibited by molecules that compete with the substrane for access to the active site.
|
|
|
Allosteric Regulation
|
A molecule binds at a site other than the active site and causes the enzyme to change shape making the active site inaccessible.
|
|
|
Aldose
|
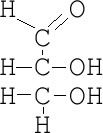
-
|
|
|
Ketose
|
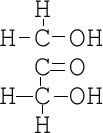
-
|
|
|
Glycosidic Linkage
|
Polymerization of simple sugars through a condesation reaction of two hyrdoxl groups.
|
|
|
Starch
|
Glucose monomers joined by glycosidic linkages.
|
|
|
Glycogen
|
Highly branched polysacchoride
|
|
|
Polysaccharide
|
"many sugars" polymers formed from linked monosaccharides.
|
|
|
Chitin
|
A polysaccharide that stiffens cell walls in plants. Also forms the skeletons of insects.
|
|
|
Phosphorylase
|
Enzyme that catalyzes the hydrolysis of glycosidic linkages in glycogen.
|
|
|
Amylose
|
Unbranched helix
|
|
|
Amylopectin
|
Branched helix
|

