![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
112 Cards in this Set
- Front
- Back
|
Define asthma.
|
Chronic inflammatory airway disorder caused by a range of innocuous stimuli with widespread, variable airflow obstruction. Reversible with time/treatment.
|
|
|
Why can a lack of wheeze be an ominous sign?
|
Lungs can be so blocked and the patient so tired that the wheeze actually stops.
|
|
|
What is hypersensitivity?
|

Immunologically driven host tissue-damaging process.
|
|
|
What are the four types of hypersensitivity?
|
Type 1 - IgE, mast cell degranulation
Type 2 - IgG/IgM complement mediated cytotoxicity Type 3 - Soluble IgG immune complex hypersensitivity Type 4 - T-cell dependent delayed hypersensitivity |
|
|
What is a typical onset for type 1 versus type 4 hypersensitivity?
|
Type 1, 1-5mins, Type 4, 4-24 hours
|
|
|
Which hypersensitivity types are involved in asthma?
|
Type 1 and type 4.
|
|
|
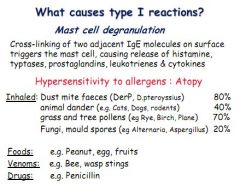
What immune event triggers the activation of mast cells?
|
Cross-linking of IgE molecules
|
|
|
What inflammatory mediators are found in mast cells, and which are preformed and which are de novo?
|
Histamine and typtases (preformed)
Prostaglandins, leukotrines and cytokines (de novo) |
|
|
Give some examples of allergens which might trigger a type 1 hypersensitivity.
|
Inhaled (pollens, spores, pet dander), foods (peanut, fruit), venom (bee/wasp stings, bites), drugs (penicillin)
|
|
|
Give examples of some inhaled allergens.
|
Pet dander, house dust mites, fungi spores, pollen.
|
|
|
Give examples of some type 1 reactions.
|
Systemic anaphylaxis, allergic rhinitis (hay fever), urticaria (skin hives), asthma
|
|
|
How would you diagnose a type 1 hypersensitivity?
|
Symptoms - rhinitis, eye streaming/conjunctivitis, asthma/wheezing, dermatitis
Skin prick test with allergen and look for wheal and flare Lab test, look for raised IgE in response to antigen (e.g. Radioallergosorbent test RAST) |
|
|
Define a type 2 hypersensitivity.
|
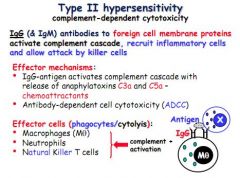
IgG and IgM antibodies respond to foreign cell membrane proteins and activate complement cascade (especially C3a and C5a), receuiting inflammatory cells and killer cells.
|
|
|
Which two elements of the complement cascade are anaphylatoxins/chemoattractants?
|
C3a and C5a
|
|
|
Give some examples of type 2 hypersensitive triggers.
|

Incompatible blood transfusion
Rhesus system in new born Autoimmune disease, e.g. diabetes type 1, or Myasthenia Gravis Haptogenic drugs |
|
|
Define a type 3 hypersensitivity and give examples.
|
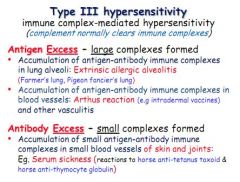
Similar to type 2 but soluble IgG mediated. Activate complement cascade, recruit inflammatory cells and killer cells. Also involves C3a and C5a. Can cause build up of antigens - antigen excess.
E.g. Pigeon fancier, arthus reaction, serum sickness |
|
|
What are some complications of antigen excess in type 3 hypersensitivity?
|
Antigen can accumulate in lung (Pigeon fanciers lung), large blood vessels (arthus reaction) or in small blood vessels in bones and joints (serum sickness)
|
|
|
Define a type 4 hypersensitive reaction (and when is it seen in asthma?)
|
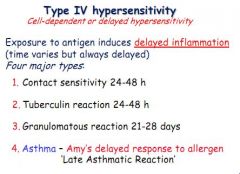
Antigen exposure induces a delayed response, independent of antibody, of 4 types:
1. Contact sensitivity 2. Tuberculin reaction 3. Granulomatous reaction 4. Asthma (late reaction) |
|
|
Type 4 reactions are said to be independent of antibody, if so, what mediates a type 4 response?
|
T-cell mediated.
|
|
|
Describe the outcome of involvement of T-cells type 1 and 2 in a type 4 reaction.
|
TH-1 - Macrophages - Tuberculin
TH-2 - Eosinophils - Asthma |
|
|
Which T-cells are involved in a type 4 hypersensitive reaction resulting in asthma?
|
TH-2.
|
|
|
What are the symptoms of asthma and when are they often worse?
|
Cough, wheezing, chest tightness, dyspnoea.
Worse at night or early morning. |
|
|
What can bring asthma on?
|
Innoculous stimuli.
|
|
|
What are the main characteristics which define asthma?
|
Reversibility, symptoms respond either over time or with treatment
Non-specific tightening to range of stimuli (cold, irritants, pollens) Intense inflammation, can cause airway remodelling in chronic cases |
|
|
What are the two types of asthma?
|
Atopic/extrinsic - definite external cause
Non-atopic/intrinsic - no definite external cause |
|
|
Define atopic asthma.
|

Atopic/extrinsic - definite external cause
Most prevalent, usually begins in childhood Diagnose with skin prick test IgE dependent, so can be diagnosed with blood test |
|
|
Define non-atopic asthma.
|
Non-atopic/intrinsic - no definite external cause
Late onset, usually adulthood, less common Skin prick test usually negative IgE independent, so usually no lab test |
|
|
Define an occupational asthma.
|

Late onset, can be atopic (flour/grain) or non-atopic
|
|
|
Define a drug-induced asthma.
|
Aspirin/NSAIDs can provoke attacks, B-antagonists can also cause attacks, contraindicated in asthma.
|
|
|
What is airway remodelling?
|
Persistent alterations to normal airway structure.
|
|
|
Describe 4 histological feature of broncho-inflammation.
|
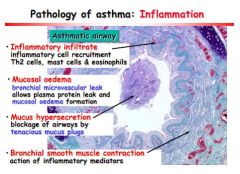
Inflammatory infiltration, TH2, mast cells and eosinophils
Mucosal oedema, leaky vessels, proteins out, oedema Mucus hypersecretion, causing airway blockage Bronchial smooth muscle contraction, by inflammatory mediators |
|
|
Describe 4 histological feature of airway remodelling.
|
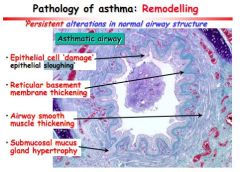
Epithelial sloughing
Thickening of reticular basement membrane Airway smooth muscle thickening Submucosal mucus gland hypertrophy |
|
|
What are the consequences of airway inflammation, remodelling and bronchiolar smooth muscle contraction?
|

Airway hypersensitivity and obstruction = asthma.
|
|
|
What centres in the pons control breathing?
|
Pneumotaxic and apneuistic
|
|
|
What centres in the medulla control breathing?
|
Dorsal and ventral respiratory group
|
|
|
What feeds directly into the pons to affect breathing?
|
Higher centres, emotions and temperature feed into the apneuistic and pneumotaxic centres in the pons.
|
|
|
What feed directly into the medulla to affect breathing?
|
Chemoreceptors (central and peripheral), Lung receptors (stretch, irritants and juxtapulmonary proprioreceptors).
|
|
|
Where are the lung stretch receptors?
|
Bronchial wall smooth muscle.
|
|
|
What is the Hering Breuer reflex?
|
When the lungs inflate, stretch receptors inhibit inspiration.
|
|
|
What are the Hering-Breuer and deflation reflexes and how are they stimuated?
|

Hering Breuer - when lungs inflate, expiration is stimulated
Deflation Reflex - when lungs deflate, inspiration is stimulated Both by stretch receptors in bronchial smooth muscle |
|
|
Where would you find juxtapulmonary 'J' receptors?
|
Alveolar or bronchial walls, near capillaries.
|
|
|
Juxtapulmonary J receptors in the lung are innervated by the vagus. What does stimulation of these receptors induce?
|

Apnoea/shallow, rapid breathing
Slow pulse, fall in BP Constriction of larynx Relaxation of skeletal muscle |
|
|
What stimulates juxtapulmonary J receptors?
|
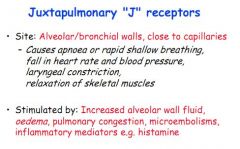
Oedema, alveolar fluid increase, microembolism, histamine release
|
|
|
Where might you find irritant receptors?
|
Throughout airways, in between epithelial cells.
|
|
|
What does stimulation of irritant receptors cause?
|

In the trachea, a cough. In the lower airways, hyperpnoea. Also bronchial and laryngeal constriction.
|
|
|
What stimulates irritant receptors?
|
Irritants! Smoke, dust, rapid inhalations/exhalations
|
|
|
What is responsible for the deep augmented breaths conducted every 15-20mins when resting?
|
Irritant receptors - they prevent lung collapse in quiet breathing.
|
|
|
What are stimulated by shortening and load of respiratory muscles to achieve appropriate tidal volumes?
|
Proprioceptive afferents.
|
|
|
Other than the Juxtapulmonary J receptors, the stretch receptors, the irritant receptors and the proprioceptive afferents and central chemoreceptors what other receptors might influence breathing?
|
Pain receptors can cause a brief apnoea
Arterial baroreceptor activation can inhibit breathing |
|
|
How can you estimate metabolic rate?
|
Production of CO2, from PCO2
Consumption of oxygen, from PO2 Production of H+, from plasma pH |
|
|
What is the effect of increased CO2 production, at what happens at the upper limit?
|
Breathing increases (ventilation) as production of CO2 increases, until >8kPa, then breathing can stop as respiratory centres become depressed.
|
|
|
What are the effects of acidosis and alkalosis on breathing?
|
Acidosis causes increased breathing, and alkalosis decreased breathing.
|
|
|
What happens as PO2 falls?
|
Breathing rate increases (peripheral chemoreceptors)
|
|
|
What is the combined effect of hypoxia and hypercapnia?
|
Synergistic, the combined effect is greater than the effect of each on their own.
|
|
|
Where are respiratory chemoreceptors located in the brain?
|
Medulla, ventrolateral surface, near exit of 9th and 10th cranial nerves
|
|
|
What do central chemoreceptors respond to?
|
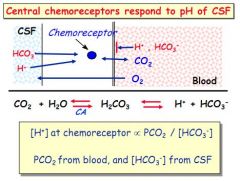
pH of the CSF - primarily dictated by arterial PCO2 NOT arterial pH
|
|
|
What factors influence the pH in the CSF?
|
PCO2 from the blood, and [HCO3-] in CSF, NOT blood pH so much
|
|
|
What factor primarily influences central chemoreceptors?
|
pH of the CSF - driven by arterial PCO2 (not arterial pH)
|
|
|
Do central chemoreceptors normally respond to PO2?
|
No.
|
|
|
Why does the fact that CSF contains little protein make it ideal for monitoring of pH by chemoreceptors?
|
Little protein = bad buffer. So small changes in PCO2 will create large changes in pH of CSF.
|
|
|
Is the response to a change in CSF pH by central chemoreceptors fast?
|
Comparitively slow, it can take up to 20 seconds.
|
|
|
How do central chemoreceptors respond to prolonged hypercapnia?
|

Adapt - CSF pH will normalise
Drive to hyperventilate removed |
|
|
How do central chemoreceptors respond to altitude?
|
Hypoxia normally creates an alkalosis, as pH normalises drive increases.
|
|
|
Where are the peripheral chemoreceptors?
|
Aortic arch and carotid sinuses.
|
|
|
What are the two cell types in carotid or aortic bodies?
|
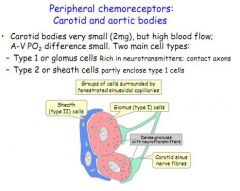
Type 1 - glomus cells, rich in axons and cell bodies
Type 2 - sheath cells, surround type 1 glomus cells |
|
|
How and why do the peripheral chemoreceptors influence breathing?
|
Sense change in PO2 and PCO2, fast
High PCO2 will increase firing, causing an increased breathing Low PO2 will also increase firing, increasing breathing |
|
|
Do peripheral chemoreceptors respond to PO2 or O2 content?
|
PO2.
|
|
|
What are the two types of sleep apnoea?
|

Neuromuscluar cause, e.g. muscular dystrophy, CAN'T BREATH
Brainstem damage e.g. Curse of Ondine, WON'T BREATH |
|
|
What neurotransmitters cause contraction of airway smooth muscle?
|
Parasympathetic tone (Vagus) - AcH on muscarinic M3 receptors
Excitatory Non-Adrenergic/Non-Cholinergic 'c-fibre' efferents - Substance P, Neurokinin |
|
|
Which three neurotransmitters cause relaxation of airway smooth muscle?
|

Sympathetics to adrenal medulla, adrenalin on B2 receptors
Sympathetic stimulation of parasympathetic ganglia Inhibitory Non-Adrenergic/Non-Cholinergic using Nitric Oxide |
|
|
Where is airway smooth muscle found, and how is it configured?
|
All levels from trachealis to bronchioles, wrapped circumferetially
|
|
|
What are the main aims of asthma therapy?
|
To minimise symptoms of airway obstruction caused by constriction of smooth muscle and inflammation.
|
|
|
How much of the drug administered by Metered-dose Inhaler reaches the lungs?
|
15-20%
|
|
|
What are the two main types of asthma drug therapy?
|
Relievers - Bronchodilators (B2-agonists), Salbutamol/Ventolin for acute symptoms, blue
Preventers - Prophylatic/Anti-inflammatories, Becotide, steroid, brown |
|
|
What are the two main types of relievers for acute asthma symptoms?
|
B2-agonists, or Anti-Cholinergics (but not if atopic)
|
|
|
What are the main types of preventers for chronic asthma symptoms?
|
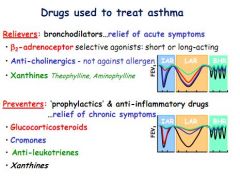
Glucocorticosteroids, cromones and anti-leukotrienes
|
|
|
What are the two types of B2-agonists and how do they work?
|
Short-acting, Salbutamol
Long-acting, Salmeterol Both work by increasing cAMP levels, causing smooth muscle relaxation, stabilisation of mast cells, and clearance of mucus due to increased ciliary frequency |
|
|
What is the main method of action of anti-cholinergics in asthma treatment?
|
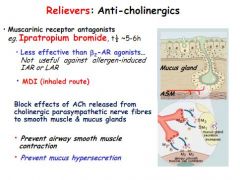
Muscarinic receptor antagonists
Not as effective as B2 agonists and don't work in allergen induced asthma Prevent bronchoconstriction and hypersecretion of mucus |
|
|
What are Xanthines, how do they work and when are they used?
|
Inhibit phosphodiesterase, which breaks down cAMP
Has both reliever and anti-inflammatory effects, but not very good Lots of side effects, so only used if B2-agonists don't work |
|
|
Do glucocorticoids work quickly?
|
No, can take an hour before any effect is seen.
|
|
|
How do glucocorticoids work and how are they used in asthma treatment?
|
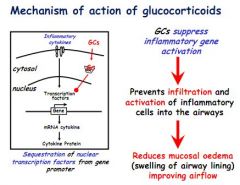
Work by suppressing gene activation in pro-inflammatory cytokines, very effective
Used long term to reduce inflammation, and in conjunction with a reliever for acute symptoms |
|
|
What are cromones and when are they used?
|
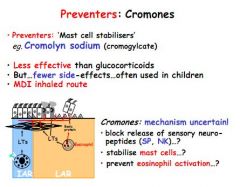
Preventers. Work by stabilising mast cells. They are not as effective as glucocorticosteroids but have few side effects so good for kids.
|
|
|
What are anti-leukotrienes, how do they work and when are they used in asthma treatment?
|

Anti-leukotrienes are preventers which stop Leukotrienes formed from arachidonic acid in cell membranes. These play a role in inflammation. Anti-leukotriences are best used with a glucocorticoid, but are also useful in asthma caused by use of NSAIDs
|
|
|
What are the common side effects of B2-agonists?
|
Muscle tremor (common), tachycardia (less common)
|
|
|
What are common side effects of anti-cholinergics?
|
Rare/none
|
|
|
What are Xanthines, how do they work, and what are their common side effects?
|
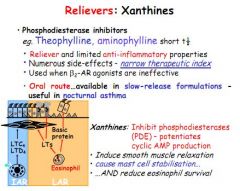
Xanthines are relievers with some anti-inflammatory effects. They work by inhibiting phosphodiesterase: Potentiate cAMP, leading to smooth muscle relaxation. Side effects include headaches, nausea, diuresis, convulsions.
|
|
|
What are common side effects of steroids?
|
Thrush, hoarseness, growth retardation, bruising, osteoporosis, thin skin, hypertension, weight gain
|
|
|
What are common side effects of anti-leukotrienes?
|
Rare. Headaches.
|
|
|
Other than drugs, what are good strategies to avoid acute asthma symptoms?
|
Reduce exposure to allergens, avoid smoke/car fumes, avoid workplace irritants and try to avoid chest infections.
|
|
|
Using %age of expected FEV-1, how are asthma drugs prescribed?
|
100% - B2 agonist reliever
80-100% - Add steroid 50-80% - Add long acting B2 agonist 50-80% - Increase dose and add xanthine/anti-leukotriene <50% - Add prednisolone (severe cases only) |
|
|
What are some of the issues in asthma adherence?
|
Preventer effect subtle so not taken
Steroid side-effects can be serious Trigger factors not avoided Symptoms not always effectively monitored Follow up appointments missed if healthy at the time Inhaler not used correctly Drugs not taken in correct proportion |
|
|
What six doctor patient factors influence adherence?
|
Quality of communication, message info etc
Relationship between dr and patient Patient beliefs about illness/treatment Situational barriers (finance, access) Mood (e.g. depression) Complexity of treatment, side-effects, is it a cure/prevention etc |
|
|
What two factors are said to influence compliance in relation to taking medication?
|
Necessity of taking medication
Concerns about what might happen if you do/don't take it |
|
|
How can adherence be improved?
|
Better communication, simple advice, comprehensible
Provide written information as well Consider from patient angle - anticipate concerns, beliefs etc Aim to collaborate in treatment |
|
|
Placebo effects are attributed to what?
|
Expectancy of effect
Conditioning from previous treatments Cognitive dissonance Reduced anxiety Endorphin release |
|
|
List some examples of lung functions tests.
|
FEV-1, CO diffusion across alveolar wall, arterial blood gases, spirometry for lung volumes.
|
|
|
What is FEV-1 and how is it measured?
|
Forced Expiration Volume (FEV) in 1 second
Measure on a Vitalograph, used to give an FEV-1/FVC ratio |
|
|
Residual volume, functional residual capacity and total lung capacity cannot be measured using a spirometre, how can these be measured?
|
Using helium dilution.
|
|
|
Functional residual capacity is raised in what condition?
|
Emphysema
|
|
|
Functional residual capacity is reduced in what condition?
|

Lung fibrosis
|
|
|
How can the FEV-1/FVC ratio be used to diagnose an obstructive or restrictive lung disease?
|
If FEV-1/FVC ratio is reduced - Obstructive
If FEV-1/FVC ratio is normal - Restrictive |
|
|
What is a normal FEV-1/FVC ratio and what might it be in a typical COPD sufferer?
|
Normal FEV-1/FVC ratio is 75-80%, in COPD this is reduced as FEV-1 is diminished by obstruction (in a restrictive disease e.g. asthma, the FEV-1/FVC ratio would be normal as both would be reduced)
|
|
|
Why is the FEV-1/FVC spirometry reading done after a bronchodilator is administered?
|
Asthma is reversible, whilst COPD/emphysema is not
|
|
|
What are the three causes of airway obstruction in COPD?
|

Chronic bronchitis - Enlarged mucus glands (inflammation) - causing productive chronic cough (diagnosed if most days over 3 months over 2 years)
Chronic obstructive bronchitis - Inflammation, scarring and obliteration of bronchioles, resulting in breathlessness Emphysema - Loss of alveolar soft tissue, causing loss of elastic recoil and breathlessness |
|
|
Why is giving oxygen to COPD sufferers dangerous?
|
Long term high PCO2 causes a greater reliance on PO2 levels for breathing control. Hyperventilation important for removal of CO2, if O2 is given, breathing slows, and PCO2 can rise, leading to coma/death
|
|
|
Why might an asthmatic wake coughing at night?
|
Inflammation of airways increases at night (various explanations), and swelling exposes c-fibres, which cause coughing
|
|
|
What is 'morning dipping'?
|
Tendency for FEV-1 to be worse in the morning, due to nocturnal irritation.
|
|
|
Why is the morning and evening good times to measure peak flow to monitor asthmatic patient's lung performance?
|
Performance is typically at it's worst morning and evening, so taking readings then gives the 'worst case scenario' which we are hoping to avoid.
|
|
|
What is status asthmaticus and what are the signs?
|
Life threatening asthma attack
Inability to complete sentences due to lack of breath Silent chest - no crackles Respiratory rate >25/min Pulse >110bpm Cyanosis Very reduced peak flow reading (<50% normal) |
|
|
What causes the Immediate Asthmatic Response and how long does it last?
|
0-20 minutes to take hold, airway narrowing caused by bronchoconstriction as mast cells degranulate releasing histamine
|
|
|
What is the Late/Delayed Asthmatic Response and how long does it last?
|
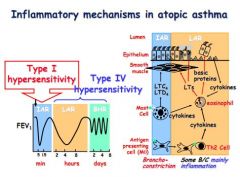
1-12 hours, airway narrowing due to mucosal swelling, oedema.
|
|
|
Why during a prolonged attack might the salbutamol inhaler cease working and what can you do to treat this?
|

Hyperventilation means drug isn't getting to lungs properly, try nebuliser
After time the delayed phase inflammation kicks in, B2-agonist not a good treatment, try oral steroids? Desensitisation can take place if B2-agonist is overused in an attack, in which case try an anti-cholinergic or xanthine |

