![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
128 Cards in this Set
- Front
- Back
|
Anatomy |
The study of the structure of the body. ( what it's made of) |
|
|
Physiology |
The study of the function of the body( how it works) |
|
|
Relationship of structure and function |
The structure determines the function a change in structure means a change in function |
|
|
The 10 characteristics of life |
Responsiveness and adaptability(irritability), movement, growth, respiration respiration, digestion, absorption, assimilation, excretion excretion, circulation, and reproduction. |
|
|
Responsiveness and adaptability |
Responsiveness indicates that the organism recognizes changes in its internal or external environment. Adaptability changes the organism's Behavior capabilities or structure |
|
|
Movement |
Distributes materials throughout large organisms; changes orientation or position of a plant or immobile animal. Movies mobile animals around the environment. Locomotion getting from point A to point B or a change in position |
|
|
Growth |
Indicates that the organism is successful it's a sign of adapting, increasing in size while maintaining shape. |
|
|
Respiration |
The exchange of gases between the body and outside the absorption and utilization of oxygen and CO2 |
|
|
Digestion |
The mechanical and chemical breakdown of food( energy,parts and nourishment)- make it small |
|
|
Absorption |
An organism's ability to selectively take in matter from the outside in. - take it in |
|
|
Assimilation |
The ability of an organism to selectively take something it's absorbed and make it a part of it. |
|
|
Excretion |
The elimination of chemical waste products generated by the organism. The ability to selectively eject matter the organism doesn't need. |
|
|
Circulation |
Movement of fluid within the organism may involve a pump and a network of special vessels also called the transport system. |
|
|
Reproduction |
1. Perpetuating a species , continuing life by continuing you 2. Cellular reproduction making more of you by making cells to maintain and repair the body. |
|
|
Needs of an organism |
Maintaining boundaries, water, food, oxygen, heat, and pressure |
|
|
maintaining boundaries |
The need of an organism for a contained environment separating what you need to stay alive from the outside examples being skin and membrane |
|
|
Homeostasis |
The ability of an organism to maintain stable and optimal internal conditions in response to changing external or internal conditions |
|
|
Negative feedback mechanism |
A change of One Direction will bring about a change in the opposite direction |
|
|
Positive feedback mechanism |
A change in One Direction brings about a change in the same direction or Perpetual change |
|
|
Levels of organization from least complex to most complex |
Chemical( atoms, molecules, macromolecules) Cells Tissues Organs Organ systems Organism |
|
|
Organelles |
Working parts found in cells larger combinations of macromolecules |
|
|
Cells |
A group of organelles working together for a common function also the smallest unit of life |
|
|
Tissues |
A group of cells working together for a common function - epithelial , Connective, muscle, and nervous |
|
|
Organs |
A group of tissues working together for a common function any group of tissues is an organ |
|
|
Organ systems |
A group of organs working together for a common function |
|
|
Organism |
A group of systems working together for a common function |
|
|
Name the major organ systems |
Integumentary, skeletal, muscular, nervous, endocrine , digestive, respiratory, circulatory , lymphatic, urinary, and reproductive. Keep in mind that the interdependence of all the systems |
|
|
The integumentary system |
Protection from environmental hazards and Temperature Control contains skin, hair, and nails |
|
|
The skeletal system |
Support, protection of soft tissue, mineral storage, and blood formation contains bones and cartilage |
|
|
The muscular system |
Locomotion, support, and heat production contains muscles and tendons |
|
|
The nervous system |
Control, communication, and coordination contains the brain, spinal cord, and nerves |
|
|
Endocrine system |
Directing long-term changes in the activities of other organ systems communicates with hormones through the blood. Contains pituitary, thyroid, parathyroid, adrenal, and pineal glands also pancreas and gonads. |
|
|
Digestive system |
Breaks down food, absorbs nutrients, and eliminates waste. Contains mouth, esophagus, stomach, intestines, gallbladder, liver, and pancreas |
|
|
respiratory system |
Gas exchange and acid-base balance. Contains lungs, bronchial tree, trachea, and nose. |
|
|
Circulatory or cardiovascular system |
Works with respiratory system to transport nutrients oxygen, hormones and waste |
|
|
Lymphatic system |
Defense against infection and disease aids circulatory brings excess fluid back. Contain lymph nodes , lymph, thymus , and spleen |
|
|
Urinary system |
Removal of metabolic waste and water salt and pH control. Contains kidneys, urethra, ureters, and urinary bladder |
|
|
Reproductive system |
Production of sex cells and hormones which help with bone strength. Female contains- ovaries, uterus, fallopian tubes and vagina. Meal contains - testes, prostate, vas deferens , and penis. |
|
|
energy |
The capacity to do work or to put matter into motion |
|
|
Kinetic energy |
Energy in motion capable of doing work |
|
|
potential energy |
Stored energy capable of doing work but not currently doing it |
|
|
chemical energy |
Energy that is stored in the bonds of chemical substances example ATP adenosine triphosphate |
|
|
Electrical energy |
The movement of charged particles |
|
|
mechanical energy |
Energy that is directly involved in moving matter |
|
|
Radiant or electromagnetic energy |
Energy that travels in waves |
|
|
Matter |
Anything that occupies space and has mass |
|
|
Mass |
The amount of material in matter |
|
|
Atom |
Smallest stable units of matter, The smallest particles of an element that still displays the chemical properties of that element |
|
|
Element |
Pure substances consisting of only the same atoms with the same atomic number |
|
|
Elements most abundant in living matter |
Ponch Phosphorus Oxygen Nitrogen Carbon Hydrogen These elements make up 97% of the human body |
|
|
Protons |
Positively charged particles found in the center or nucleus of an atom |
|
|
Neutrons |
No charge or neutral found in the nucleus |
|
|
Electrons |
Negative charge, swirling about the nucleus |
|
|
Atomic number |
The number of protons, the number of protons determines the element |
|
|
Atomic mass |
Number of protons plus the number of neutrons equals atomic mass |
|
|
Atomic weight |
The average of atomic masses of all isotopes of an element |
|
|
Isotopes |
Atoms with the same atomic number but a different atomic weight meaning a different number of neutrons. Please behave the same as any other atom of that element |
|
|
Ions |
A positively or negatively charged particle |
|
|
Cation |
A positively charged particle meaning it lost an electron |
|
|
Anion |
A negatively charged particle meaning it gains an electron |
|
|
Rules of chemical bonding |
1. Bonding occurs because Matter wants to be in its most stable state 2. Only electrons in the outermost shell are involved in bonding 3. Atomic stability equals eight electrons in the outermost shell or a full Shell |
|
|
Ionic bond |
Two atoms are bonded together because of charge attraction from Gaining or losing an electron |
|
|
covalent bond |
Atoms share electrons and are bonded to achieve stability. Double and triple bonds are possible |
|
|
Hydrogen bonds |
Due to the existence of polarity for polar covalent bonds the positive end of one molecule attracts to the negative end of another |
|
|
Solid |
Maintains volume and shape |
|
|
Liquid |
Constant volume but no fixed shape |
|
|
Gas |
Neither a constant volume nor a fixed shape |
|
|
Properties of water |
No other chemical exists as a solid, liquid, and gas in nature water makes up 60 to 70% of the volume of most living cells. Water is a polar molecule meaning it is very soluble. It is the universal solvent. Water has a high heat capacity Water has a high heat of vaporization Water is an important reactant in many chemical reactions Water forms a protective barrier around organs Water is a good lubricant |
|
|
Acid |
Anything that releases hydrogen ions(H+) in a solution. Often called proton donors |
|
|
Base or alkaline |
Anything that releases hydroxyl ions (OH-) in a solution. Called proton acceptors |
|
|
Salt |
Any cation other than H+ that gets released and any anti on other than OH- |
|
|
PH scale |
What is neutral at 7- Anything below is acid with a low ph And anything above 7 is a base with a high ph. |
|
|
Buffers |
Chemicals that health reasons for large changes in PH |
|
|
The effects of alkalinity and acidity on the nervous system of the body |
Acidity depresses the nervous system causing it to be harder to excite the nerves Alkalinity makes a nurse easily excitable having seizures Etc |
|
|
Synthesis |
Anabolic Bond forming reaction |
|
|
Decomposition |
Catabolic a bond breaking reaction breaking a molecule into smaller ones |
|
|
Exchange |
Both synthesis and decomposition happening at the same time |
|
|
Endergonic reactions |
Most anabolic reactions are energy absorbing |
|
|
Exergonic reaction |
Are releasing energy most catabolic reactions release energy |
|
|
Activation energy |
The amount of energy that must be invested into a reaction before it will happen |
|
|
Enzymes |
Are chemicals that lower the activation energy of a reaction be speeded up but are not used up |
|
|
Organic molecules |
Any molecule that contain carbon and hydrogen together 4 types are carboxylic acid comma Amino, hydroxyl, and phosphate |
|
|
Carbohydrates |
Are made of carbon hydrogen and oxygen Are relatively sellable in water can change shape and are 1 - 2% of cell Mass Major functions: a major source of cellular fuel, help guide cell interaction or communication Karma the liver can take them and make amino acids for protein. |
|
|
Monosaccharides |
One simple sugar, the building blocks of a larger carbohydrates examples are glucose galactose and fructose |
|
|
Isomers |
I need chemicals with the same chemical formula but a different chemical structure example glucose and fructose both have C6H12O6 formula but different shape |
|
|
Disaccharides |
Two simple sugars together their primary function is energy to use them we need to break it down to be able to absorb for example breaking down sucrose results in glucose and fructose comma breaking down lactose results in glucose and galactose comma breaking down maltose results in glucose and glucose |
|
|
Polysaccharides |
Many stacked sugars |
|
|
Glycogen |
A polysaccharide animals store excess carbohydrates very branched just glucose |
|
|
Starch |
a polysaccharide plants store excess sugar coil more less branched just glucose |
|
|
Cellulose |
A polysaccharide used in plant cell walls it's a long straight chain of glucose enzymes cannot digest it requires teeth to break it in order to get inside |
|
|
Glucose |
C6H12O6 |
|
|
Lipids |
AKA fat made of CHO (P)carbon hydrogen oxygen and sometimes phosphorus there is very few oxygen are there insoluble in water and soluble in organic substances |
|
|
Glycerides |
neutral fats are composed of a glycerol molecule attached to one two or three fatty acid chains |
|
|
Glycerol |
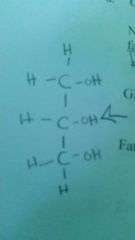
C3h8o3 |
|
|
Fatty acid chain |
Acid bound to a chain of carbon and hydrogen could be saturated which would be no double bonds or unsaturated which would have a double or triple bond |
|
|
Phospholipids and glycolipids |
Glycerol + 2 fA chains+ phosphate group |
|
|
Eicosanoids |
Lipids that came from arachadonic acid |
|
|
Prostaglandins |
Local Mini hormones made locally by cells to affect things locally |
|
|
Steroids |
Four rings of carbon made from cholesterol |
|
|
steroid hormones |
Cortisol and aldosterone |
|
|
Anabolic steroids |
building steroids most are based on testosterone can cause health problems such as sterility psychosis behavioral issues and glowing inside the body |
|
|
Proteins |
are made of amino acids CHON (SP) carbon hydrogen oxygen nitrogen and sometimes phosphorus and sulfate. They are water-soluble the most fundamental part of you |
|
|
structural proteins |
Help maintain a shape or form such as hair tendons ligaments and spider silk |
|
|
Contractile proteins |
Produce movement such as muscles or cilia |
|
|
Storage proteins |
Store things such as albumin in egg white |
|
|
Defensive proteins |
fight and kill pathogens such as antibodies |
|
|
transport proteins |
carry things throughout the body example hemoglobin |
|
|
Messenger protein |
chemical Messengers such as hormones from the thyroid |
|
|
Enzyme protein |
speed up reactions but are not used up they lower the activation energy |
|
|
Amino acids |
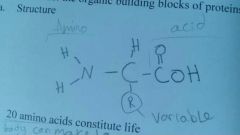
are the building blocks of proteins there are 20 amino acids that constitute life 10 are made in our body can we find from our diet |
|
|
Peptide bonds |
Bond between carboxylic and nitrogen end |
|
|
Polypeptides |
10 or more peptide bonds |
|
|
peptide bonds to proteins |
Proteins are 50 or more peptide bonds |
|
|
primary structure |
Is determined by the sequence the amino acids are linked in |
|
|
Secondary structure |
such as the alpha helix or pleated sheet are caused by hydrogen bonding between atoms |
|
|
Tertiary structure |
The complex overall 3D shape a protein takes is caused by interactions between groups or surrounding water |
|
|
Quaternary shape |
Two or more polypeptides to form a protein complex example of globular or fibrous protein |
|
|
Denaturation |
Altering the shape of a protein which means it will lose its function |
|
|
Substrate |
chemical acted upon by the enzyme |
|
|
active site |
The part of the enzyme that binds to the substrate like a lock and key |
|
|
Things that affect enzyme activity |
Temperature, salt concentration, and acidity or pH |
|
|
Enzyme helpers |
Enable the enzyme to work at the active site |
|
|
Cofactor |
An enzyme helper that is an ion or molecule that attaches to the active site before it will work |
|
|
Coenzyme |
An enzyme helper that is organic molecules that act as co-factors |
|
|
high-energy compound |
job is to provide energy for the cells common compounds are ATP ADP and amp |
|
|
Nucleic acids |
are the largest molecules in the body composed of chonp examples are DNA and RNA |
|
|
nitrogenous bases |
in DNA adenine pairs with thymine and cytosine pairs with guanine comma in RNA adenine pairs with yourself and cytosine pairs with guanine |
|
|
surface area to volume ratio |
A cells surface area to volume ratio must be large because a cell must be able to get enough nutrients inside of it so that all of its parts function, as well as the able to remove enough waste so that it doesn't drown in its own toxins |

