![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
14 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
What is the main difference between a pure substance and a mixture? Name an example of each. |
Pure substance: made up of only one kind of matter Mixture: a combination of pure substances |
i.e. gold and soil |
|
|
What is a chemical change? |
A chemical change always results in the formation of a new substance or substances. |
i.e. when zinc metal and hydrochloric acid are mixed, they undergo a chemical change that produces two new substances. |
|
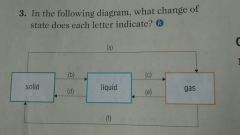
|
(a) Sublimation (b) Melting (c) Evaporation (d) Freezing (e) Condensation (f) Deposition |
|
|
|
Low density polyethylene is a plastic that can be stretched somewhat without breaking it. Why is this property useful for disposable shopping bags? |
Because then the bags won't rip from the pressure/weight of groceries like paper bags do. |
|
|
|
Explain why particles of water in the air can form frost on a cold window. |
When the water particles comes in contact with a cold window, the process of heat transfer by contact (conduction) occurs. Now the water particles change from gas to liquid and clings to the glass (adhesion). If the glass is cold enough, the particles become a solid (freezing). |
|
|
|
What are two physical changes that cooling a hot substance may result in? |
Condensation, freezing. |
|
|

|
(a) conductivity (b) malleability (c) lustre (d) hardness |
|
|

|
(a) P (b) C (c) C |
|
|
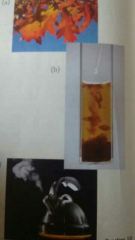
Identify the processes shown in the following photos as chemical or physical changes. |
(a) P (b) C (c) C |
|
|

|
(a) Solution (b) Mechanical mixture (c) Suspension |
|
|
|
Metal foams with 75 to 95% air. What effect does this have on the density of metal foam compared to solid metal. |
Since air is less dense than solid metal, metal foams are less dense overall. |
|
|
|
How can the application of heat result in a chemical change. |
A chemical change Always result in the formation of a new substance or substances. |
I.e. when baking powder is heated it undergoes a chemical change that results in the production of carbon dioxide gas. |
|
|
If water freezes inside of a building's water pipes, the pipes may burst. Explain why this happens. |
When water freezes, its particles expand as they freeze. As the ice expands, it pushes water toward the closed faucet. This causes an immense amount of water pressure to build between the ice blockage and the faucet — eventually, the pipe ruptures under the pressure. |
|
|
|
Some types of clear plastic can be used to make lunches list three important properties of plastic that make it suitable for use and eyeglass lenses |
Hardness malleability transparency |
|

