![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
95 Cards in this Set
- Front
- Back
|
|
|
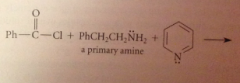
|
|
|
|
|
|
|
Mechanism
|
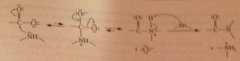
unless another base added, starting amine acts as base in last step
|
|
|
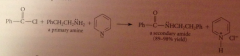
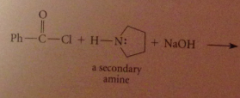
Using a tertiary amine such as pyridine or triethylamine does not interfere w amide formation by another amine bc
|
a tertiary amine itself cannot form an amide
|
|
|
Schotten-Baumann technique
|
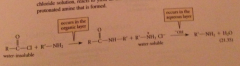
reaction is run with an acid chloride in a separate layer over aq. soln NaOH = the amine reacts to yield an amide & aq. NaOH extracts & neutralizes the protonated amine that is formed
|
|
|
Hydrolysis of the acid chloride by NaOH is avoided bc acid chlorides
|
are typically insoluble in h2o, so not in direct contact w h2o-soluble hydroxide ion
|
|
|
Either 2 equiv of amine must be used or
|
an equivalent of base must be added to effect the final neutralization
|
|
|
|
|
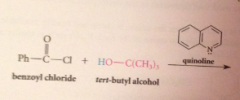
|
|
|
|
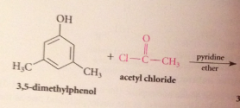
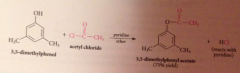
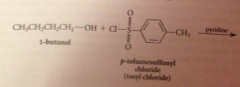
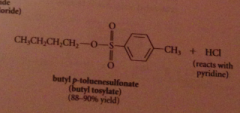
Why doesn't the HCl liberated not need to be neutralized?
|
alcohols & phenols are not basic enough to be extensively protonated by the acid but pyridine used to neutralize the HCl
|
|
|
|
|
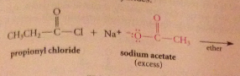
Even though carboxylate salts are weak nuc, acid chlorides
|
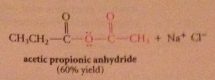
reactive enough to react w carboxylate salts to give anhydrides
|
|
|
Use of acid chlorides in organic synthesis
|

|
|
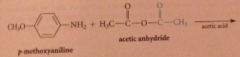
|
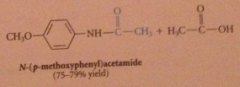
anhydrides react w nuc to yield amide, ester etc
|
|
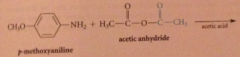
Why is this only used w inexpensive & readily available anhydrides?
|
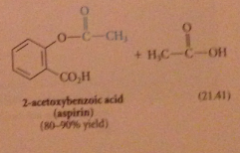
one equivalent of parent acid is wasted as a LG
|
|
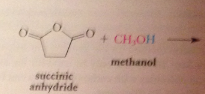
exception: from cyclic anhydrides are formed
|
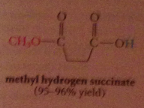
half-esters and half amides
|
|
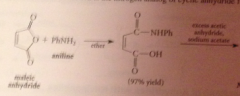
|
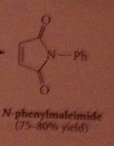
|
|
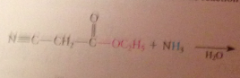
Reaction of esters w ammonia
|
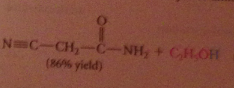
yields amides
|
|
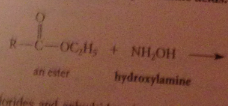
|
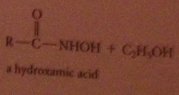
Esters are much less reactive toward amines and alcohols, but sometimes useful
|
|
|
Hydroxamate test
|
hydroxamic acid products easily recognized bc form highly colored complexes w ferric ion
|
|
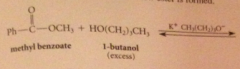
when an ester reacts w alcohol + acid or alkoxide w base
|
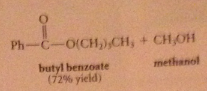
An ester is formed
|
|
|
Transesterification
|
the conversion of one ester into another by rxn w an alcohol
|
|
|
equilibrium constant typically
|
~1 because neither ester strongly favored @ equilibrium
|
|
|
The reaction is driven to completion by the use of
|
an excess of the displacing alcohol or by removal or a relatively volatile alcohol by product as it is formed
|
|
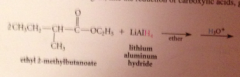
Lithium aluminum hydride reduces all CA derivatives
|
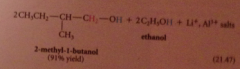
reduction of esters gives primary ROHs: by product methanol or ethanol typically discarded
|
|
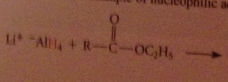
|
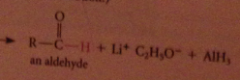
The active nucleophile is the hydride ion
|
|
|
Mechanism 2
|
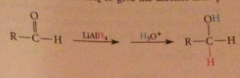
Aldehyde reacts rapidly w LiAlH4 to give the alcohol after protonolysis
|
|
|
NaBH4 is ___ reactive than LiAlH4
|
less: reduces aldehydes & ketones but reacts sluggishly w most esters - can be used to reduce aldehydes & ketones selectively in presence of esters
|
|
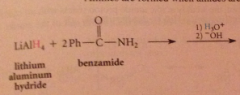
|
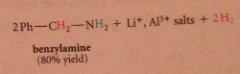
|
|
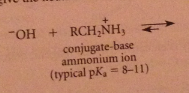
|
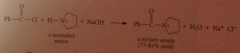
The excess of acid that is typically used converts the amine, which is a base, into its conjugate-acid ammonium ion & hydroxide is required to neutralize the ammonium salt & give neutral amine. h2o can be used but acid more convenient, so neutralization necessary
|
|
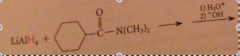
|
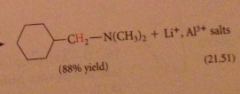
preparation of secondary/tertiary amines from secondary & tertiary amides
|
|
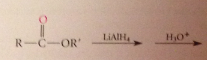
Ester reduction
|
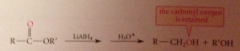
carbonyl oxygen lost as LG
|
|
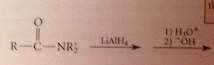
Amide reduction
|

|
|
|
Why is the carbonyl oxygen lost in amide reduction?
|
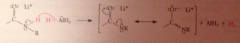
The weakly acidic amide proton reacts w equiv. hydride, strong base, to give AlH3 & lithium salt of amide
|
|
|
Mechanism 2
|
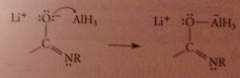
The lithium salt of the amide, a LB, reacts with the LA AlH3
|
|
|
Mechanism 3 - the resulting species is an active hydride reagent, conceptually like LiAlH4 and can deliver hydride to the C=N double bond
|
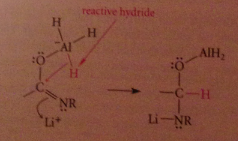
The O-AlH2 group then lost from tetrahedral intermediate bc less basic than other possible LG resulting in imine
|
|
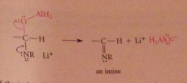
the C=N of the imine undergoes nuc add w H-
|
addition of acid to the rxn mixture converts add intermediate into an amine by protonolysis, then into conj. acid ammonium ion, which is neutralized to free amine when -OH is added
|
|

|
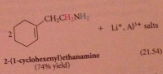
isolation of the neutral amine requires addition of -OH @ conclusion of rxn
|
|
|
Nitrile reduction mechanism
|
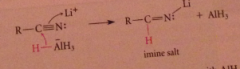
first nuc add
|
|
|
Nitrile reduction mechanism 2
|
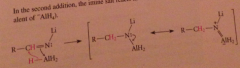
|
|
|
In the resulting derivative both the N-Li and the N-Al bonds are very polar and the N has anionic character
|
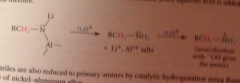
both bonds are susceptible to protonolysis, so an amine then ammonium is formed when aq. acid is added to the rxn mixture
|
|
|
Mechanism
|

Raney nickel is a type of nick-Al alloy
|
|
|
What is the intermediate in the rxn?
|
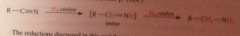
Imine, which is hydrogenated to the amine product
|
|
|
Any synthesis of a CA can be used as part of an amine synthesis, but the amine prepared must have the form
|
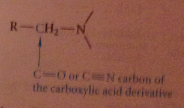
|
|
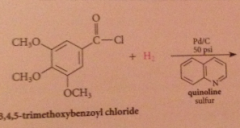
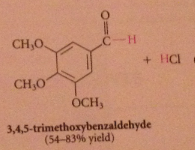
Rosenmund reduction
|
|
|
|
What are catalyst poisons?
|
Amines, sulfides, prevents further reduction of aldehyde product
|
|
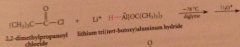
|
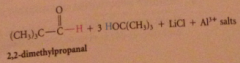
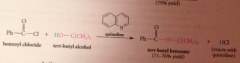
Hydride derived by replacement of 3 H of LiAH4 by tert-butoxy groups => less reactive reagents obtained
|
|
|
poor reactivity of hydride
|

rxn of LiAlH4 w tert-butyl alcohol stops after 3 moles of alcohol consumed, remaining hydride reduces only most reactive func groups
|
|
|
reagent reacts preferentially w acid Cl over product aldehyde bc
|
acid Cl are more reactive than aldehydes toward nucs
|
|
|
LiAlH is so reactive that
|
it fails to discriminate btwn aldehyde + acid Cl group, so reduces acid chlorides to primary alcohols
|
|
|
reaction of LiAlH w CA or ester involves aldehyde intermed but product is
|
primary alcohol bc aldehyde intermed is more reactive than the acid or ester
|
|
|
Li tri tertbutoxy Al H reduction of acid Cl can be stopped @ aldehyde bc
|
acid cl more reactive than aldehydes - product aldehyde in competition w remaining acid Cl for hydride reagent - more reactive acid Cl consumed before aldehyde has chance to react
|
|
|
relative reactivities of carbonyl cmpds
|
nitriles < amides < esters, acids << ketones < aldehydes < acid chlorides (MOST REACTIVE)
|
|
|
relative reactivity is determined by the stability of
|
each type of carbonyl cmpd relative to TS for add/sub
|
|
|
esters are less reactive than aldehydes bc
|
stabilized by resonance
|
|
|
acid Cl destabilized by
|
e-attracting polar effect of Cl
|
|
|
TS energies for nuc sub rxn of acid Cl lowered by
|
favorable LG properties of Cl
|
|
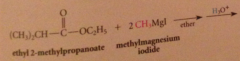
|
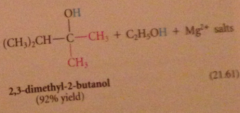
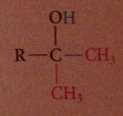
tertiary alcohol formed after protonolysis
|
|
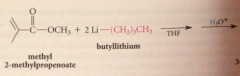
nuc acyl sub followed by addition
|
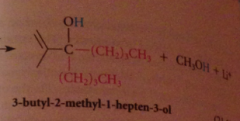
2 equiv of organometallic reagent react per mole ester, second alcohol produced in rxn
|
|
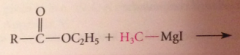
ketone intermed not isolated bc
|
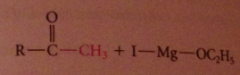
|
|
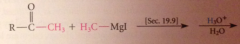
|
ketones are more reactive than esters towards nuc reagents, so reacts w 2nd equiv of grignard reagent to form magnesium alkoxide, which after protonolysis gives the alcohol
|
|
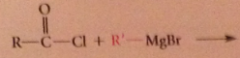
bc acid cl more reactive than ketones
|
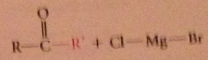
reaction of acid cl w grignard reagent can give ketone w/o further rxn of ketone itself
|
|
|
transformation is diff to achieve in practice w/o careful control of rxn conditions bc
|
grignard reagents are very reactive (hard to prevent further rxn of product ketone w grignard reagent to give alcohol)
|
|
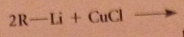
|

lithium dialkylcuprates are less reactive than grignard and organolithium reagents - typically react readily w acid Cl, aldehydes, epoxides, slowly w ketones, not at all w esters
|
|
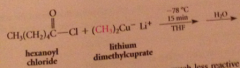
ketones do not react further bc
|
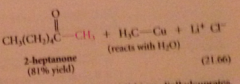
they are much less reactive than acid Cl toward lithium dialkylcuprates
|
|
|
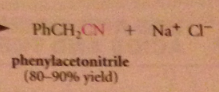
|
|
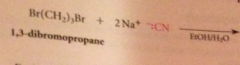
|
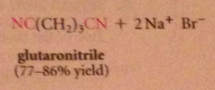
|
|
|
nitriles can be particularly useful as an intermediate step in the preparation of a CA bc
|
nitriles are prepared from cmpds other than CA derivatives
|
|
|
most important occurrence of amides in nature
|
proteins: polymers in which a-amino CA units connected by amide linkages
|
|
|
nylon
|
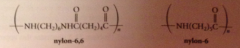
group of polymeric amides/ polyamides
|
|

|
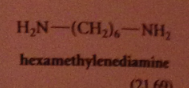
|
|
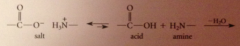
|
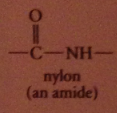
|
|
|
more vigorous conditions needed than rxn of amine w ester bc
|
amine is basic, equil on L favors salt
|
|
|
In salt amine is protonated and therefore
|
not nuc, and carboxylate ion unreactive toward nuc
|
|
|
small amt amine and CA in equil w salt
|
react when salt is heated, pulling equil to R
|
|
|
starting material for nylon-6
|
e-caprolactam
|
|
|
both adipic acid and e-caprolactam are prepared from
|
cyclohexanone, prepared by oxidation of cyclohexane
|
|
|
cyclohexane comes from
|
petroleum (example of dependence of important segment of chem economy on petroleum feedstocks)
|
|
|
Condensation polymer
|
formed in a rxn that liberates a small mlc
|
|
|
nylon as a condensation polymer
|
formation of each amide bond accompanied by loss of small mlc H2O
|
|
|
addition polymer
|
polyethylene is an example: one mlc adds to other w/o loss of mlclr fragment
|
|
|
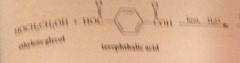
polyesters
|
condensation polymers derived from rxn diols & dicarboxylic acids
|
|
|
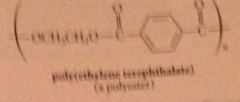
|
|
|
polyester prod. depends on raw materials derived from
|
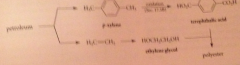
petroleum
|
|
|
waxes, fats and phospholipids are all important naturally occurring
|
ester derivatives of fatty acids
|
|
|
wax
|
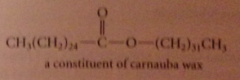
ester of a fatty acid & fatty alcohol, primary alcohol w long unbranched C chain
|
|
|
fat
|
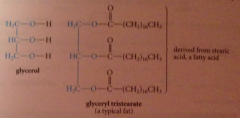
ester derived from mlc of glycerol & 3 mlcs fatty acid
|
|
|
acyl groups in a fat
|
may be same or different
|
|
|
unsaturation
|
form of 1+ cis db
|
|
|
saturated fats
|
no db, typically solids i.e. lard
|
|
|
unsaturated fats
|
contain db, often oily liquid i.e. olive oil
|
|
|
Saponification
|
treatment of fats w NaOH or KOH for glycerol & Na or K salts of fatty acids
|
|
|
phospholipids
|
esters of glycerol
|
|
|
structural diff. btwn fat & phospholipid
|
in phospholipid, one glyceryl primary hydroxy group esterified to a polar phosphoric acid derivative rather than to a fatty acid
|

