![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
29 Cards in this Set
- Front
- Back
|
describe carboxylic acids |
- have a carboxyl group consisting of a carbonyl group and a hydroxyl group attached to the terminal carbon - names using the suffix -oic acid -methanoic acid is the simplest carboxylic acid and it is used in bee and ant stings - ethanoic acid is the acid in vinegar, important in the chemical industry about 6.5 million tonnes are used each year worldwide |
|
|
describe the reactivity of carboxylic acids |
results in part from the polarisation of its bonds |
|
|
describe the difference between weak and strong acids |
in strong acids all molecules ionise but in weak acids only a fraction of molecules ionise |
|
|
why are carboxylic acids stable |
the negative charge is delocalised across the carboxylate group. the delocalisation is represented by adding a seconfd dotted line to the carbon oxygen bonds which are both equivalent |
|
|
what do carboxylic acids react with |
sodium carbonate and sodium hydrogencarbonate reacion with ethanoic acid and sodium carbonate 2CH3COOH + Na2CO3 --> 2CH3COONa + co2 +H2O reaction of methanoic acid and sodium hdrogencarbonate HCOOH + NaHCO3 --> HCOONa + CO2 + H2O the formation of bubbles of carbon dioxide mkes these reactons useful as a test for carboxylic acids |
|
|
describe carboxylic acids reacting with alkalis |
RCOOH + NaOH --> RCOONa + H2O |
|
|
how are esters formed |
by the reaction of carboxylic acids and an alcohol in the precence of concentrated acid |
|
|
how are esters named |
they all take the form of alcohol group followed by carboxylate group all esters end in -oate |
|
|
name some uses of esters |
solvents perfumes plasticers natural and artificial flavouring vegatable oils and animal fats |
|
|
describe ester hydrolysis |
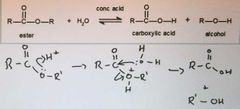
carried out in acid or alkaline conditions you get different products from each set of conditions |
|
|
describe alkaline hydrolysis |

|
|
|
desribe saponification |

|
|
|
describe biodiesel |
biodiesel is a mixture of methyl esters and long chained carboxylic acids it isa transesterification reaction where the ester groups are changed the fats /oils are reacted with methanol and potassium hydroxide in the presence of a catalyst using it is carbon neutral and it is a renewable source |
|
|
describe acylation |
the process in which an acyl group(RCO-) is introduced to another molecule by an acylating agent acid derivatives contain an acyl group and are derived from carboxylic acids. they have the general formula RCOZ and include esterss,acids chlories and acid anhydrides. acid derivatives are important acylating agents due to their polarised carbonyl group. |
|
|
what does the speed of acylation depend on |
- the reactivity of the nucleophile according to its ability to donate its lone pair to an electron deficient carbon - the electron releasing or attracting power of Z which affects the magnitude of the + charge on the carbonyl carbon - how easily Z is lost |
|
|
describe acid achlorides |
containa cghlorine atom attached to the acyl group. named with the suffix -oyl; the term chloride is also added. |
|
|
describe acid anhydrides |
prepared bythe reaction of 2 carboxylic acids resulting the in elimination of a molecule of water. named with the suffix -oic and the term anhydride is also added. |
|
|
describe the reactivity of acid chlorides. |
- the oxygen and chloride atos attached tothe acyl carbon are groups that withdraw electron density - as a result the c-o and the c-cl bonds are both polar with a delta positive sign on the carbon atom -the means the acyl carbon atom is susceptible to attack by nucleophiles resculting in the subsitution of the chloride atom |
|
|
describe the reactivity of aacoid anhydrides |
the same as acid chlorides but it results in the subsitution of the carboxylate ion |
|
|
What is the addition elimination mechanism of acid chloride and water |
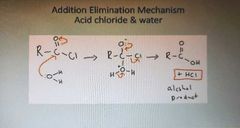
|
|
|
What is the addition elimination mechanism for acid chloride and alcohol |
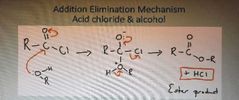
|
|
|
What is the addition elimination mechanism of acid chloride and ammonia |

|
|
|
What is the addition elimination mechanism of acid chloride and a primary amine |
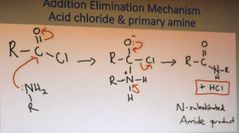
|
|
|
Describe using acid chlorides vs acid anhydrides |
- acid anhydride is favoured because acid chlorides are difficult to handle as they react readily with water and it produces corrosive HCl -acid anhydrides on the other hand are cheaper to produce, not as moisture sensitive, less corrosive and produce carboxylic acids which are safer than HCl. |
|
|
What is the addition elimination mechanism of acid anhydrides and water |
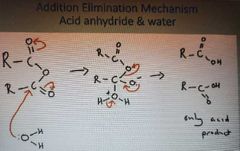
|
|
|
What is the addition elimination mechanism of acid anhydrides and alcohol |
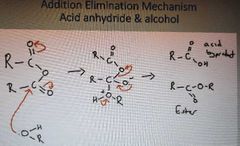
|
|
|
What is the addition elimination mechanism of acid anhydrides and ammonia |
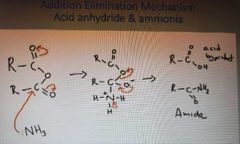
|
|
|
What is the addition elimination mechanism of acid anhydrides and primary amines |
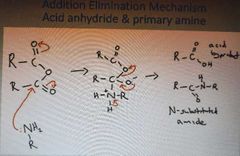
|
|
|
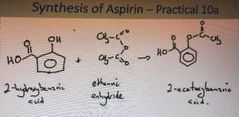
What is the synthesis of aspirin |
|

