![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
23 Cards in this Set
- Front
- Back
|
electrons are arranged in |
energy levels |
|
|
energy levels a.k.a |
energy shells |
|
|
shells contain.. |
sub-shells (sub-levels) |
|
|
each shell.sub-shell is made up of... |
electron orbitals |
|
|
each electron orbital can hold... |
2 electrons |
|
|
The electron pair in each orbital have _____ spins |
Opposite (1 clockwise, 1 anti-clockwise) |
|
|
Orbitals are.. |
regions of space that electrons are most likely to be in |
|
|
Sub-level types... |
s, p, d, f |
|
|
state the: - number of orbitals in sub-level s -maximum number of electrons in sub-level s |
- 1 orbital - 2 electrons |
|
|
state the: - number of orbitals in sub-level p -maximum number of electrons in sub-level p |
- 3 orbitals - 6 electrons |
|
|
state the: - number of orbitals in sub-level d -maximum number of electrons in sub-level d |
- 5 orbitals - 10 electrons |
|
|
state the: - number of orbitals in sub-level f -maximum number of electrons in sub-level f |
- 7 orbitals - 14 electrons |
|
|
state the sub-levels (in order of filling) and maximum number of electrons in energy level 1: |
- s - 2 electrons |
|
|
state the sub-levels (in order of filling) and maximum number of electrons in energy level 2: |
- s, p
- 8 electrons |
|
|
state the sub-levels (in order of filling) and maximum number of electrons in energy level 3: |
- s, p, d
- 18 electrons |
|
|
state the sub-levels (in order of filling) and maximum number of electrons in energy level 4 / >4: |
- s, p, d, f - 32 electrons |
|
|
Maximum number of electrons for energy level n ... |
2n^2 |
|
|
Rules for filling orbitals: |
- fill from lowest to highest energy - if 2 or more orbitals of the SAME energy are available, e- are NOT paired until they have to be - if 2 e- occupy the same orbital, their 'spins' must be opposite |
|
|
Fill orbitals form ______ to _______ energy (Auf Bau Principal) |
lowest to highest |
|
|
if 2 or more orbitals of the same energy are available... (Hund's Rule) |
electrons are NOT paired in orbitals until they have to be |
|
|
If 2 electrons occupy the same orbital... (Pauli Principal) |
their spins must be opposite |
|
|
Order to fill... |
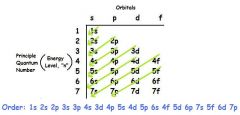
|
|
|
4s rules --> (ions)
|
4s both fills and emptys first |

