![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
76 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
Entropy: Though Experiments |
Mechanical Equilibrium |
Ball on the top of the incline: potential energy (gravitational) due to position. When released, potential energy will convert to heat (friction) and to kinetic energy as it rolls down). At rest, potential energy equals zero, thus the ball is at a mechanical equilibrium. |
|
|
Experiment # 2 |
Dissolve NaOH in water Solution becomes warm - heat is released |
|
|
|
Experiment #3 |
Dissolve Solid NaNO3 in water Solution becomes cool, heat is absorbed |
|
|
|
Experiment # 4 |
Two identical chambers (A&B) connected by a stopcock |
|
|
|
What is Entropy |
A thermodynamic property that is the measure of a systems thermal energy per unit temperature that is unavailable for doing useful work. |
|
|
|
What is thermodynamic entropy? |
Is more generally define from a statistical thermodynamics viewpoint in which the molecule nature of matter is explicitly considered. Another thought experiment. |
|
|
|
The N number of molecules in this system the probability that at equilibrium all N molecules will be found in … ? |
Left chamber is (1/2)^N |
Entropy : Probabilistic Explanation |
|
|
The probability that the molecules will be … |
Distributed equally between chambers will increase |
Entropy: Probabilistic Explanation |
|
|
Entropy is the measure of ? |
“Randomness” or “Disorder” |
|
|
|
The second law of thermodynamics: |
All natural processes occur only in one direction—— one that leads to minimum potential energy |
|
|
|
Entropy change can be mathematically expressed as: |
dS = dH/T or S = H/T |
|
|
|
The Third Law of Thermodynamics: |
The entropy of a perfect material at absolute zero is exactly equal to zero. |
|
|
|
It is impossible to cook any system to T equals to 0 K, so.. |
S is always positive. |
|
|
|
What is Gibbs Free Energy |
Combines these two factors and it is a state function: |
G = H - TS
This has an energy component and an entropic component |
|
|
What is incremental form? |
Used most commonly to determine the change in free energy |
G = H - T S |
|
|
For a process to occur spontaneously, the G term should have a ? |
Negative value (energy minimization) |
|
|
|
Equilibrium is the position where … |
G = 0 |
|
|
|
The process during which the “heat content” of the system remains constant is known as an … |
Adiabatic process |
Application of Thermodynamic Principles in Pharmacy |
|
|
‘The entropy of a perfect material at absolute zero is exactly equal to zero.’ This statement is a description of … ? |
3rd law of thermodynamics |
|
|
|
‘The entropy of a perfect material at absolute zero is exactly equal to zero.’ This statement is a description of … ? |
3rd law of thermodynamics |
|
|
|
For an EXOTHERMIC reaction, which one of the following is true? |
Change in enthalpy (H) is negative |
|
|
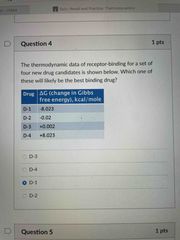
Front (Term) |
D-1 |
|
|
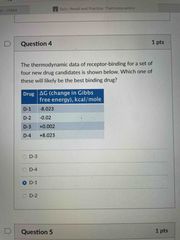
Front (Term) |
D-1 |
|
|
|
Chemical Kinetics and Drug Stability Introduction |
A pharmacist interrupts information related to drug stability provided by the manufacturer and relays it to the patients. |
He/she recommends proper storage and use of dosage forms. |
|
|
What are the 5 types of stability ? |

Back (Definition) |
|
|
|
Physical stability |

Back (Definition) |
|
|
|
Chemical stability |
Influenced by chemical structure, environmental and formulation factors |
|
|
|
Oxidation-Reduction |
Major cause of drug instability |
|
|
|
Autoxidation: Functional Groups |

Back (Definition) |
|
|
|
How can Photolysis be prevented? |
Suitable packaging in amber colored bottles Cardboard Outers Aluminum foil over wraps |
|
|
|
Oxidation: Prevention |
Use carefully chosen antioxidants Minimize exposure Eliminate trace metals Light resistant Refrigeration and maintaining pH |
|
|
|
What is hydrolysis? |
Drugs containing ester, amide, imide, imine, and lactam functional groups are prone to hydrolysis. - Often a pH-dependent reaction involves solvolysis, a process in which a drug molecule interacts with a water molecules to yield breakdown products. - Buffers - used to maintain the pH at which drug stability is optimum. - The amount of water present in formulation affects the rate of hydrolysis. |
|
|
|
What is inter-ionic compatibility and decarboxylation? |
Chemical instability caused by addition of a large ionic species with a charge opposite to that of a drug.
Decarboxylation - Involves removal of the carboxyl group from a drug molecule |
|
|
|
Racemization |
Process of changing from an optically active compound into a race mix compound or optically inactive mixture of ‘R’ and ‘S’ forms |
|
|
|
Epimerizarion |
A chemical reaction leading to formation of an epimer of the active compound. Epimers are diastereomers with difference in chirality only at one sterogenic center |
|
|
|
Drug stability importance |

Back (Definition) |
|
|
|
Microbial Contamination: Sources |

Back (Definition) |
|
|
|
Expiration date : purpose |
Loss of active ingredient and potency due to decomposition of drug Decomposition products can be harmful Physical changes can decrease the efficacy |
|
|
|
Reaction rate |
The velocity with which a reactant(s) undergoes a chemical change |
|
|
|
Reaction rate: Factors |

Back (Definition) |
|
|
|
Law of Mass Action |

Back (Definition) |
|
|
|
Law of Mass Action |

Back (Definition) |
|
|

Order of reaction: Zero Order Reactions
|

Back (Definition) |

Order of reaction: Zero Order Reactions |
|
|
Order of Reaction: First Order Reactions |

Back (Definition) |
|
|

Order of Reaction: Second Order Reactions |

Back (Definition) |

|
|

Order of Reaction: Second Order Reactions |

Back (Definition) |

|
|

Rate constants |

Back (Definition) |
Half life is the time required for the reaction to reach half the initial concentration of the reactant ( 1/2 A0) |
|
|
What is Shelf life? |
Time required for 10% of the drug to degrade 90% of drug remains intact. |
|
|

Front (Term) |

Back (Definition) |
Ea is always positive The value of Ea does not change with temperature |
|
|
Stability testing |

Back (Definition) |
|
|
|
Shelf life estimation: Q10 Method |
Method based on Ea Allows estimation of shelf-life for a product under different storage conditions |
t90(T2) = t90(T1)/Q10 (T/10) Where, Ea is the energy of activation, R is the gas constant and T is the absolute temperature |
|
|
Q10 Method (Cont.) |

Back (Definition) |
|
|
|
Other factors affecting reaction rates and stability |
PH , solvents, presence of additives/excipients, surfactants |
|
|
|
The statement, “In all spontaneous processes there is an increase in the entropy of the system and it’s surrounding,’ represents the … ? |
2nd law of thermodynamics |
|
|
|
“The entropy of a perfect material at absolute zero is exactly equal to zero.” The statement is a description of… ? |
3rd law of thermodynamics |
|
|
|
For an EXOTHERMIC reaction, which one of the following is true? |
Change in enthalpy (H) is negative |
|
|
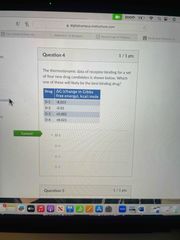
Front (Term) |
D-1 |
|
|
|
The statement, “In all spontaneous processes there is an increase in the entropy of the system and it’s surrounding,’ represents the … ? |
2nd law of thermodynamics |
|
|
|
Solubility: Introduction |
“The concentration of a solute when the solvent has dissolved all the solute it can at a given temperature.”
Or
“The concentration of solute in a saturated solution at equilibrium.” |
Solubility is an intrinsic property of molecule and can only be modified by modifying the molecule chemically. Dissolution is an extrinsic property and can be a influenced by complication, particle size, surface properties, and solubilization enhancement techniques. |
|
|
What is Miscible? |
When both solute and solvent are liquids and they form a solution over a wide concentration range (one phase) |
|
|
|
What is Saturated solution? |
One in which dissolved solute is in equilibrium with the solid phase. |
|
|
|
What is Saturated solution? |
One in which dissolved solute is in equilibrium with the solid phase. |
|
|
|
What is Unsaturated solution? |
A solution in which solute is present in a concentration below the concentration required for its complete saturation at a given temperature. |
|
|
|
What is Saturated solution? |
One in which dissolved solute is in equilibrium with the solid phase. |
|
|
|
What is Unsaturated solution? |
A solution in which solute is present in a concentration below the concentration required for its complete saturation at a given temperature. |
|
|
|
What is Supersaturated solution? |
- Contains more solute than a saturated solution - It is very unstable, and solute generally precipitates out (due to equilibration) ups on agitation, seeding crystals, or scratching the container side. |
|
|
|
Solubility Expressions (USP) |

Back (Definition) |
|
|
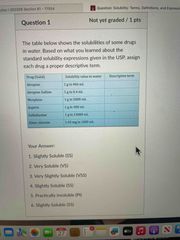
Front (Term) |
Answers are above |
|
|

Types of Solvents |

Back (Definition) |

|
|
|
Intrinsic Solubility (S0) |
Solubilization is an equilibrium process. At saturation solubility, the drug in solution is an equilibrium with solid drug
S0 = K = [Drug]solution/[Drug]solid
For non-electrolytes, S0 is same as the total solubility |
|
|
|
Factors affecting solubility/dissolution |
1. Chemical structure of the solute 2. Crystalline Vs. Amorphous forms 3. Polymorphs 4. Micronization 5. Temperature |
|
|
|
Factors Affecting Solubility/Dissolution |

Back (Definition) |
|
|
|
Factors Affecting Solubility/Dissolution |

Back (Definition) |
|
|
|
Factors Affecting Solubility/Dissolution |

Back (Definition) |

|
|

Front (Term) |

Back (Definition) |

|
|

Front (Term) |

Back (Definition) |
|

