![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
214 Cards in this Set
- Front
- Back
|
are viruses visible under standard light microscopes?
|
genearlly no, but pox virus can just be resolved
|
|
|
resolving power of TEM
|
.25nm
|
|
|
resolving power of SEM
|
10nm
|
|
|
how is the high resolutoin of EM made possible
|
a straem of electrons which have packages of energy associated with very short wavelengths
|
|
|
TEM- viruses can be viewed through
|
negative staining and thin sectioning
|
|
|
negative staining
|
staining the virus preparation with a non penetrating stain- the stain will stain the background and not the virus.
|
|
|
most frequently used negative stain
|
phosphotungstic acid
|
|
|
simplest and most rapid method of detecting and recognizing virus particles
|
negative staining
|
|
|
in order for negative staining to be successful:
|
the amount of virus in a specimen must be high
|
|
|
negative stain EM permits the designation of detected virus particles into its respective ____ _____ based on size, shape, structure
|
family classification
|
|
|
Immunoelectron microscopy
|
rapid serological diagnostic test using EM
|
|
|
describe the IEM method
|
virus preparation is mixed with virus specific antibodies (such as virus specific monoclonal Ab). These MAb can be labeled with or attached to small iron or gold particles. The ab causes the viruses to clump together, making it easier to visualize the virus particles in the midst of opaque round gold particles. the iron or gold labeled ab will appear as dark dots and are easy to discern
|
|
|
thin sectioning method
|
used to prepare fixed and embedded tissues or cells for TEM
|
|
|
advantages of thin sectioning method
|
permits visualization of the site of virus replication and maturation in host cells
|
|
|
most commonly used thin sectioning fixatives
|
glutaraldehyde, cacodylate, osmium tetroxide
|
|
|
ultramicrotome
|
used to section epoxy resin blocks, into sections as thin as .030um
|
|
|
what stains are used with thin sectioning method
|
lead citrate and uranyl acetate (electron opaque materials)
|
|
|
disadvantages of thin sectioning method
|
takes time, requires personell with specialized skills
|
|
|
for SEM, what needs to be done to the specimen?
|
coated with metal, usually gold
|
|
|
in contrast to TEM, SEM has the capacity of
|
3D imagine
|
|
|
disadvantage of SEM
|
only the surface can be observed, resolution limited to about 10 nm
|
|
|
influenza subtypes
|
16 HA subtypes, 9 NA subtypes
|
|
|
avian influenza natural hosts
|
wild birds- duck, gull, shorebirds. Virus rarely causes disease
|
|
|
all type A influenza viruses are thought to originate from
|
wild birds
|
|
|
reassortment
|
a genetic recombination even observed with influenza virus infection
|
|
|
swine flu incubation period
|
short
|
|
|
HA protein is cleaved into Ha1 and HA2 subunits by host
|
proteases
|
|
|
cleavage of HA is necessary for virus to be
|
infectious (necessary to release fusion domain)
|
|
|
HA has a receptor binding site for the _____ receptor
|
sialic acid
|
|
|
the fusion domain becomes active when
|
pH is lowered in endosome (conformation changes)
|
|
|
LPAI
|
low pathogenic avian influenza
|
|
|
emergence of HPAI
|
LPAI H5/H7 virus transmitted to poultry -> LPAI virus circulates in poultry with mild dz -> LPAI mutates to HPAI with severe disease
|
|
|
HPAI causes_____ and ____ signs
|
CNS and respiratory
|
|
|
HPAI can cause ____ and ____- on the heart and proventriculius
|
petichiation and echymosis
|
|
|
substitution mutation
|
replacing one base with another
|
|
|
where do viruses grow
|
in living cells only
|
|
|
three host systems commonly used to grow viruses
|
animals, eggs, cell cultures
|
|
|
explant cultures
|
organ cultures, maintaining fairly normal histological organization to study viral pathogenicity
|
|
|
cell cultures
|
propagation of rapidly growing ells without regard to preserving cellular organization. Forms a monolayer of cells.
|
|
|
culture type best suited for and most widely used for propagating viruses
|
cell culture
|
|
|
primary cell culture
|
first generation cell cultures obtained from "live" animal organs. Tissue is incubated in trypsin that breaks up tissue into suspension of single cell
|
|
|
newly isolated viruses from infected animals will grow better in ___ than in ____ cultures
|
primary, than in cell line cultures
|
|
|
cell line
|
transformed/cancer cells that can be propagated and sub cultured indefinitely
|
|
|
examples of cell lines
|
HeLa, Vero, crandall feline kidney and MDBK
|
|
|
MDBK
|
madin darby bovine kidney
|
|
|
most popular way to isolate and grow viruses in laboratory
|
monolayer cell line cultures set in flasks and plates
|
|
|
sub culturing
|
in order to keep propagating the cell cultures, the cell monolayer must be broken into individual cells and transferred to new tissue culture flask
|
|
|
trypsin-versene
|
used to break up the cell monolayer into individual cells
|
|
|
material most commonly used to grow tissue culture cells
|
plastic, treated with a chemical that increases the adhesion of the cells to the plastic
|
|
|
MEM
|
minimal essential media- salts, certain AA, vitamins, purines, pyrimidines, hormones, etc at an optimum osmotic pressure. MUST BE BUFFERED.
|
|
|
a common buffer added to MEM
|
sodium bicarb
|
|
|
tissue culture cells with bicarb buffer must be
|
incubated in a CO2 atmostphere.
|
|
|
HEPES
|
an organic buffer, effective at capturing excess hydrogen ions produced by the growing tissue culture cells
|
|
|
FBS
|
fetal bovine serum,
|
|
|
____ must be added to MEM
|
serum
|
|
|
how can tissue culture cells be preserved?
|
freezing at -70c of lower; indefinitely in liquid nitrogen. Need protective additive (glycerin or DMSO), proper cooling rate and rapid thawing
|
|
|
CPE
|
cytopathic effect
|
|
|
what is CPE
|
cell death and other changes caused by the replication of viruses in cell culture
|
|
|
CPE of parainfluenza
|
syncytial formation
|
|
|
cell changes that can be observed with light microscope include
|
cell destruction, inclusion bodies, syncytia,
|
|
|
hemagglutinins
|
glycoproteins that attach to rBCs
|
|
|
blind passages are usually required before ___ is observed
|
CPE
|
|
|
non cytopathic virus
|
a virus that grows without ever causing any observable CPE (strains of BVDV, etc)
|
|
|
two ways of quantifying virus
|
direct particle count, viral infectivity assay
|
|
|
viral infectivity assay
|
dilutions of the stock virus are inoculated into a test system
|
|
|
quantitative assay
|
einfectivity is quantified by counting the number of infective centers, such as plaques, pocks or foci
|
|
|
plaque assay
|
widely used in cell cultures to quantify viruses
|
|
|
plaque
|
a localized region of cell lysis resulting from the cell to cell spread of virus replicating in a monolayer
|
|
|
do all viruses produce plaques?
|
no
|
|
|
PFU
|
plaque forming unit
|
|
|
PFU
|
a term that describes the infectivity of the original viral suspension (one PFU = one infectious virus particle)
|
|
|
Quantal assay
|
only registers the presence or absence of infection. "all or none" assay
|
|
|
ID50
|
median infective dose
|
|
|
LD50
|
lethal dose
|
|
|
tissue culture infective dose
|
TCID50
|
|
|
ELD50
|
embryo lethal dose
|
|
|
reed and muench method
|
used to calculate 50% end point in quantal assay
|
|
|
Poisson distribution
|
if 100 TC wells are infected with prep that contains 1 PFU per ml, only 67% of the wells will become infected
|
|
|
Herpesvirus growing in MDBK cells- do you need to stain to see plaques?
|
no
|
|
|
pox virus lesions
|
intracytoplasmic inclusion bodies
|
|
|
bolinger's bodies
|
pox virus inclusions
|
|
|
what is unique about pox virus inclusions?
|
it is a DNA virus that causes inclusion in the cytoplasm
|
|
|
adenovirus inclusion in hepatocytes
|
basophilic IN in
|
|
|
BRD is initiated via a combo of
|
stress factors, virus infection, secondary bacterial infection (pasteurella hemolytica)
|
|
|
BRD clinical signs
|
unexpected death, dyspnea, cough, nasal discharge, inappetance, anorexia, fever, pneumonia
|
|
|
virses that have been associated with BRD
|
BHV1, IBRV, PI3, BRSV, BVD, etc
|
|
|
characteristics of BVD
|
60 nm, RNA, enveloped, +++ growth in TC, +/- CPE
|
|
|
characteristics of PI3
|
150nm, RNa, enveloped, ++++ growth in TC, HAd, synctia/IB
|
|
|
characteristics of BRSV - bov resp syncytial virus
|
150nm, RNa, enveloped, + growth in TC, syncytia
|
|
|
BHV-1
|
150 nm, DNA, enveloped, ++++ growth in TC, +/- IB
|
|
|
BAV- bovine adeno virus
|
100 nm, DNA, +++growth in TC, +/- HaD, IB
|
|
|
AHV-1: acelaphine herpes virus
|
150 nm, DNA, enveloped, +/- growth in TC
|
|
|
BReoV- bovine reovirus
|
70 nm, RNA, IB
|
|
|
BRhinoV
|
25nm, RNA
|
|
|
BCov- coronavirus
|
150 nm, RNA, enveloped, +/- growth in TC
|
|
|
inoculation onto TC cells: procedure
|
insert swab into snap cap tube containing transport medium. Place tube on ice. Add .5 ml of virus prep to MDBK monolayer flask and swirl to distribute. Incubate for ~48 hours and then s tain
|
|
|
Hemadsorption test procedure
|
pour off the media from the infected flask. Add 1 ml to saline, rotate to disperse, and then pour off the saline. Swirl, pour out media. Repeat washing. Add 2ml RBC suspension - incubate 30 minutes. Pour out RBC, add 5 ml saline, gently swirl and pour off saline. repeat washing.
|
|
|
stain the monolayer: procedure
|
add 5 mls of fixative to flask. Incubate 3 min. Pour off fixative. Add 5 mls of Solution 1. Wait 2 minutes. Pour off solution. Add 5mls of solution II. Wait 2 minutes. Pour off solution. Rinse gently with water. Pour off water. Add 2 mls of water to flask to prevent cells from drying out
|
|
|
most widely used tests to view viral antigen directly in infected animals tissue and/or on virus isolated in TC cells
|
fluorescent antibody test and IHC/IPX
|
|
|
FA test
|
can observe virus specific Ab which are tagged to a fluorescent dye, bound to viral Ag. Requires a fluorescent microscope
|
|
|
how does a fluorescent microscope work
|
emits UV light, which is adsorbed by the fluoresnt dye
|
|
|
most widely used fluorescent dye
|
fluorescein isothyocyanate (FITC)
|
|
|
advantages of FA test
|
rapid, highly sensitive, and precise
|
|
|
direct FA test
|
the virus specific ab is directly conjugated with FITC dye. Test is carried out on a microscope slide to which the tissue section or smear has been fixed. The labeled MAb (conjugate) is added and allowed to react with the RV Ag present in the brain tissue. Slide is rinsed then examined
|
|
|
IFA
|
two step reaction using two different Ab. The first Ab is a virus specific Ab that is not labeled. (ie, rabbit anti-RV Ab). The second is an FITC labelled Ab that recognizes and binds to the first Ab (goat anti rabbit FITC labeled Ab). The virus specific unlabeled Ab is added first adn allowed to react with the viral Ag in the tissue. Excess Ab is removed by rinsing. FITC labeled anti species globulin is then added and allowed to react.
|
|
|
in the direct method the FITC labeled Ab binds directly to the
|
Ag
|
|
|
in the indirect method the FITC labeled Ab does not bind directly to the Ag but
|
recognizes another antibody
|
|
|
for the FA test, viral infected tissues or infected tissue culture cells have to be ____ before addition of the virusa specific Ab
|
fixed
|
|
|
what is the usual fixative for the FA test
|
acetone for about 10 minutes
|
|
|
IHC is very similar to IFA except that
|
1) the FITC is replaced by the enzyme peroxidase. 2) the enzyme precipitates a substrate. 3) the slide is viewed with ordinary light microscope
|
|
|
enzyme most commonly used to conjugate the Ab
|
horse radish peroxidase (HRP)
|
|
|
another frequently used enzyme to conjugate the Ab
|
alklaline phosphatase
|
|
|
the primary virus specific Ab (usually a MAb) used in the IHC procedure is rarely ____
|
conjugated
|
|
|
the secondary Ab Is the ____ Ab
|
conjugated
|
|
|
how does the enzyme in IPX work?
|
catalyzes a reaction, which converts a soluble substance in the substrate solution into an insoluble precipitate, causing the precipitate to accumulate in the immediate vicinity of the viral Ag
|
|
|
what can IHC be performed on
|
tissue sections, either fresh frozen or formalin fixed
|
|
|
IHC procedure
|
primary Ab is added and allowed to react with tissue. Excess is washed off, secondary conjugted Ab is added and allowed to react. Tissue is rinsed, substrate solution added. Then counterstained with hematoxiliin and observed.
|
|
|
if the tissue is positive for a virus, the precipitate will only form
|
within the infected cells
|
|
|
advantage of IHC
|
the location of the virus wihtin the infected tissue can be determiend precisely
|
|
|
VNT can only be performed if
|
the virus has alreasdy been isolated in TC cells
|
|
|
basic VNT procedure
|
virus is mixed with virus specific antibodies (antiserum) and then added to the cell culture and the monolayer is observed for CPE
|
|
|
VNT procedure
|
a vial of the virus harvested from the tissue culture is mixed with anti-FCV Ab and a second vial is mixed with anti FHV Ab. After mixing and incubating for 1 hour, the mixture is put on separate tissue culture cells and observed for CPE.
|
|
|
HI/HAD-I
|
hemagglutination inhibition and hemadsorption inhibition
|
|
|
cell cultures infected with certain viruses have the ability to ____ RBC
|
adsorb
|
|
|
the phenomenon of ____ is caused by RBC sticking to virus specific glycoproteins called ______
|
hemadsorption; hemagglutinins
|
|
|
in HA, viruses bind RBC in solution and
|
agglutinate them
|
|
|
viruses that cause HA will generally also cause
|
HAd
|
|
|
hemadsorption inhibition pros/cons
|
very rapid, can identify virus in about 1 hour. But, not very many viruses cause HAd
|
|
|
how can hemadsorption be prevented
|
virus specific antibodies bind to the hemagglutinins on the cell membrane before RBC are added- prevents the RBC from adsorbing or sticking to the infected cells
|
|
|
HI can only be performed if
|
the virus has alreasdy been isolated in TC cells
|
|
|
HI test procedure
|
virus prep is added to an antibody solution that is thought to be specific for that virus. (Ie, if you suspect PI3, use antiPI3 virus ab solution), is added to virus solution. Incubate 60 minutes. Then add RBC. Wait 1 hour before reading resutls-- look for button formation or veil formation
|
|
|
ELISA
|
enzyme linked immunosorbent assay
|
|
|
the ELISA is ____ and ____
|
highly sensitive and specific
|
|
|
ELISA use
|
can be used to identify and quantitate viral specific Ag that has been solubilized and attached to the bottom of the plastic well or to membranes
|
|
|
with an elisa, the viral antigen must be
|
solubilized first
|
|
|
ELISa can be used to capture and
|
quantitate viral ag in serum or other body fluids
|
|
|
ELISA is based on the principle that
|
an Ab can be coupled with an enzyme and still retain both its immunological and enzymatic activity
|
|
|
enzymes primariliy used for ELISA
|
alkaline phosphatase or horseradish peroxidase
|
|
|
PCR detects
|
the presence of viral nucleic acid in the sample
|
|
|
the importance of the PCR lies in its ability to
|
1) amplify very small amounts of viral DNA or RNA from samples. And 2) test a large number of samples through automation
|
|
|
in order to perform a PCR ____ that can recognized the specific viral genome have to be available
|
primers
|
|
|
qRT-PCR
|
real time PCR
|
|
|
real time PCR uses
|
fluorescent labeled primers, is quantitative
|
|
|
true or false: false positives are uncommon in PCR
|
FALSE
|
|
|
when can PCR false negatives occur
|
if the virus mutates so that the primer can no longer recognize the mutated genome
|
|
|
PCR amplifes ___ only
|
DNA
|
|
|
RT-PCR
|
reverse transcriptase PCR
|
|
|
RT-PCR procedure
|
the genome of rna viruses have to be transcribed into a dna molecule. The enzyme that transcribes the viral rna is the reverse transcriptase
|
|
|
AGID
|
agar gel immuno diffusion
|
|
|
VNT is virus type ___
|
specific
|
|
|
VNT can tell you which ___ infeted the animal
|
serotype
|
|
|
AGID can tell you that an animal has been infected but not
|
which serotype
|
|
|
does seropositive mean infection?
|
no, can be result of of vaccination, past infection, maternal Ab, etc
|
|
|
typically, serological diagnosis requires the collection of _________
|
paired serum samples taken 3-4 weeks apart (Acute and convalescent)
|
|
|
seroconversion
|
acute sample is seronegative, convalescent sample is seropositive
|
|
|
what does seroconversion indicate
|
the disease is due to an infection
|
|
|
a significant increase in titer indicates
|
recent or ongoing infection
|
|
|
____ can help rapidly establish a diagnosis with isolation is not possible
|
serology
|
|
|
what can serology do that negative virus isolation cannot?
|
definitively rule out infection
|
|
|
examples of common serological tests
|
VNT, ELISA, HI, HAd-I, AGID, western blot, radioimmunoassay
|
|
|
when should a sample be collected from a live animal, if it is to have the greatest success at viral isolation?
|
early during the course of infection
|
|
|
when should a sample be collected at necropsy?
|
as soon as possible after death, because autolysis inactivates many viruses
|
|
|
how should samples submitted for virus isolation be shipped?
|
cold on ice, or frozen
|
|
|
what should be collected to determine viremia?
|
blood in EDTA, citrate, or heparin
|
|
|
should blood samples be frozen for shipment?
|
no
|
|
|
impression smears
|
can be obtained from live animals or from cut surfaces at necropsy. Submit air dried for virus detection, alcohol fixation for cytology, cold acetone fixation for FA test
|
|
|
how do you detect virus by histopathology?
|
from the characteristics of the lesions, or by IPX
|
|
|
most important serological test
|
elisa
|
|
|
what common test Is still run by AGID?
|
Coggins
|
|
|
virus detection tests
|
virus isolation, FA, IPX, HA, ELISA, EM
|
|
|
most important test to detect presence of virus in tissues and impression smears
|
FA test
|
|
|
direct FA
|
fluorescent labeled Ab added that will detect and bind to viral Ag in tissue
|
|
|
indirect FA
|
specific Ab added that will bind to the viral Ag, followed by fluorescent labeled Ab that will recognize and bind to the first Ab
|
|
|
importance of IPX
|
can be used on formalin fixed tissues, allows one to study viral pathogenesis
|
|
|
Resolving power of TEM compared to LM and eye
|
EM is 1000x LM. LM has resolving power of .25um, human eye is .25mm
|
|
|
Immunoelectron microscopy basic procedure
|
virus prep mixed with monoclonal Ab. MAb labeled with small iron/gold particles. Ab causes viruss to clump together- easier to virualize
|
|
|
three systems for propagating viruses
|
animals, embryonated egg, tissue and cell cultures
|
|
|
why would you choose embryonated egg to isolate influenza instead of TC cells?
|
the embryonated eggs have the protease necessary to cut the hemaglutinin
|
|
|
primary TC vs cell line TC
|
primary is first generation obtained from animal organ, whereas cell lines are transformed cells. Primary can be transformed into cell line
|
|
|
what TC did we use during lab
|
Madin Darby Bovine kidney cell line
|
|
|
MEM?
|
minimal essential medium. Basis for all cell culture media
|
|
|
FBS?
|
fetal bovine serum- added to MEM at a level of 5-10% by volume
|
|
|
how do you passage a virus?
|
the primary cell culture monolayer is only viable for 10-14 days. Needs to be subcultured.
|
|
|
what is a blind passage?
|
"Transfer of some material from an inoculated animal or cell culture that does not exhibit evidence of infection, to a fresh animal or cell culture;; passage of an infectious agent through an experimental animal or medium without there being any evidence, clinical or cultural, that the agent is present.
|
|
|
Quantitative vs quantal viral assays
|
quantal only registers the presence of absence of infection, cannot count the number of infectious viral particles
|
|
|
what is key to a quantitative assay?
|
dilution of the sample
|
|
|
In negative staining, where does the stain stay?
|
on the virus, in between the capsomeres (in valleys/spaces rather than on the sample itself)
|
|
|
positive staining
|
the protein picks up the stain, and the sample itself is stained.
|
|
|
AGID stands for
|
agarose gel immuno diffusion
|
|
|
• Advantages of negative staining in diagnosis of viral diseases and limitations of this procedure regarding viral diagnosis
|
Negative staining provides the simplest and most rapid method of detecting and recognizing virus particles. Negative staining requires high quantities (106-107 virus particles) of virus within the specimen. Negative staining allows the virus particle to be sorted into its family classification by size, shape, and structure.
|
|
|
• Understand negative staining and know the stain used to perform this procedure
|
Negative staining is the most common technique used for determining the size and shape of viruses. Method involves mixing of virus preparation with an electron-dense material such as phosphotungstate. This mixture is sprayed onto grids to be observed under EM. The viruses are viewed as light objects against a dark background. Surface structures of viruses can be demonstrated by this technique because the negative stains can penetrate gaps in the viral structure
|
|
|
does negative staining involve TEM or SEM?
|
TEM
|
|
|
• What is immuno-electron microscopy (IEM) and why use it in viral diagnosis?
|
Immuno-electron microscopy is rapid serological diagnostic testing using EM. The virus preparation is mixed with virus specific antibodies such as viral specific monoclonal antibodies. These monoclonal antibodies are attached to small iron or gold particles. The antibody causes the viruses to clump together instead of being normally scattered all over the carbon film allowing the visualization of the virus particles easier in the midst of opaque round gold particles. The iron or gold labeled antibody will appear as dark dots and are easy to discern.
|
|
|
• Know the 3 systems used for propagating viruses – why would you choose embryonated eggs to isolate influenza virus instead of tissue culture cells?
|
The three host systems used to grow viruses are animals, chicken embryos (eggs), and cell cultures. Some viruses may be grown in all three systems, while others may be more selective and grow only in one system. Influenza virus is difficult to grow (nearly impossible) in TC cells.
|
|
|
• Know the difference between primary tissue culture (TC) and cell line TC
|
Primary cell culture is the first generation cell cultures derived from cells obtained from animal organs such as kidneys, testicles, etc. They can not be cultured indefinitely. They will grow to form a monolayer and will remain viable for 10-14 days.
Cell line tissue culture is comprised of heteroploid cells in chromosomal configuration that can be sub-cultivated indefinitely. Monolayer and suspension cultures can be prepared with these cells. Monolayer cell line cultures set in flasks and plates are the most popular way to grow viruses in laboratories. |
|
|
• How do you passage viruses and what is a “blind passage”?
|
Passaging virus is transferring virus from one infected flask to another to increase adaptability. A blind passage is the transferring of virus through at least 3 flasks to allow the virus to adapt and to detect the presence of a virus.
|
|
|
• Under what condition would you perform a quantal assay instead of a quantitative PFU assay?
|
A quantal assay would be performed instead of a quantitative PFU assay because the virus does not form plaques.
|
|
|
• A virus preparation has 10^9 virus particles per milliliter when measured by EM direct virus particle count. However, a quantitative assay detects only 10^8 infectious particle per milliliter (108 PFU/ml) in the same preparation.
o What is the percentage of non-infectious virus particles in that preparation? o Is this percentage of non-infection particles within the normal range? o Explain the source of or basis for these non-infectious particles |
10^8/10^9 = 10% infectious particles or 90% non infectious particles.
Yes, it is within normal range The non-infectious particles were either infectious but have since been inactivated during growth or preparation, genetically defective or lack a complete genome, or have “empty” capsids |
|
|
o Differentiate between seroconversion versus a significant rise in Ab titers
|
Serological diagnosis of virus infection typically requires the collection of paired serum samples taken 3-4 weeks apart. These are called an acute and a convalescent sample. Seroconversion is when the acute sample is seronegative and the convalescent sample is seropositive indicating that the disease is due to an infection with the virus being tested for.
A significant increase or rise in titer is a fourfold or greater rise in serum antibody titer between the two samples that indicates a recent or ongoing infection with the virus being tested for. |
|
|
IEM
|
Immuno-electron microscopy makes use of the same antibody and monoclonal antibody that can be performed directly on infected animal’s tissue and/or on virus isolated in TC cells. Immuno-electron microscopy is available within hours, requires high viral concentration of at least 100, 000 virus particles per gram of tissue, moderate sensitivity and high specificity, and can detect virus that cannot be cultured easily.
|
|
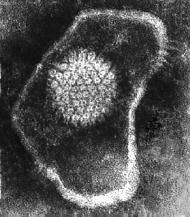
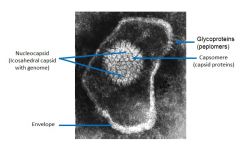
Make sure you can ID:
1. Envelope 2. Glycoproteins (peplomers) 3. Icosahedral capsid (when present) 4. Capsid proteins (capsomeres) 5. Nucleoproteins (NP) 6. Viral genome. |
|
|
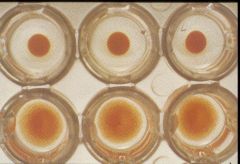
Be able to ID wells with hemagglutination
|
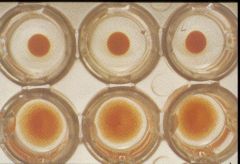
Bottom have hemagglutination
|
|
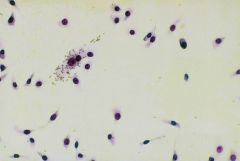
What is this test called?
|
HAd inhibition
|
|
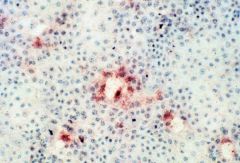
What test is this?
|
immunoperoxidase
|
|
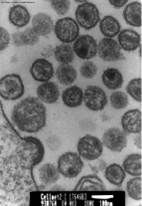
what type of staining is this
|
positive
|
|
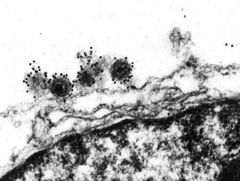
what type of microscopy is this?
|
immunoelectron microscopy
|
|
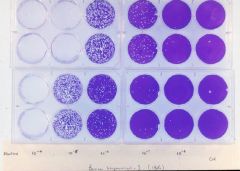
Calculate PFU
You must first dilute the virus preparation 10 fold and make subsequent dilutions – e.g. 1:10, 1:100 or 10-2, 10-3 , all the way to 10-8 or 1:100,000,000 Each well received 1 ml of the virus diluted preparation Counting the plaques - ~20 plaques at 10-7 if we count the plaques in both wells |
Therefore, we have 20 plaques forming units (PFU) in 2 ml of the virus preparation (1 ml/well) that was diluted 1:10,000,000 or 10-7
How many PFUs do we have in 1 ml of the original preparation? Answer: 20 X107 PFUs in 2 ml = 10 x 107 PFUs in 1.0 ml = 108 = 100,000,000 PFUs |
|
|
what is the virus titer?
|
is the reciprocal of the dilution that causes CPE in at least 50% of the infected wells (that is because you do not get 50% each time)
|
|
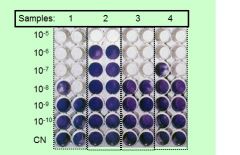
Calculate TCID50 for each sample
|
sample 1 contains 108 TCID50 in 100 ul of virus preparation
Sample 2 contains 105 TCID50 in 100 ul of virus preparation Sample 3 contains 107 TCID50 in 100 ul of virus preparation Sample 4 contains 107 TCID50 in 100 ul of virus preparation |
|
|
which tends to be higher- TCID50 or PFU?
|
TCID50
|
|
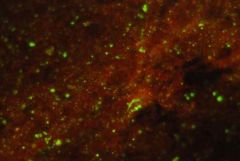
What test is this?
|
fluorescent antibody
|
|
|
what do you use to fix a sample for FA test?
|
acetone
|
|
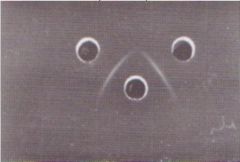
What test is this?
|
AGID
|
|
|
Most striking difference in the CPEs formed by herpes and parainfluenza?
|
first virus (Herpes) forms discrete holes. you also see some stranding where cells stretch down into the middle of plaques (due to cells fusing together). The second virus (parainfluenza) didn't have obvious nice-looking holes, but the CPE fused the cells to form large syncitia--the plaques were larger and more haphazard clumps (as opposed to perfect holes).
|

