![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
45 Cards in this Set
- Front
- Back
|
Chemical Bond |
The respective forces that hold groups of atoms together:
• Ionic - electrostatic attraction between oppositely charged ions. • Metallic - electrostatic attraction between M⁺ˣ and delocalised e⁻. |
|
|
Classification of Bonding |
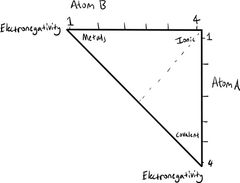
Bond triangle: Based on the electronegativities of the involved atoms. |
|
|
Covalent Bonding |
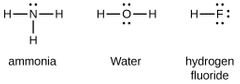
Is the sharing of e⁻ between atoms to reach a lower E. state. Resulting in AO overlap of similar E. lvls. with the same symmetry around the bond axis |
|
|
Ionic Bonding |
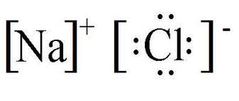
Chemical bonding that arises from the electrical attraction between an electro +VE atom (cation) and an electro -VE atom (anion). |
|
|
Metallic Bonding |
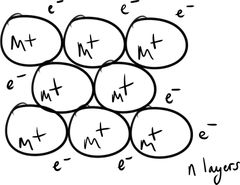
Metallic bonding results from the attraction between M⁺ˣ and the surrounding delocalised e⁻ (sea of electrons). |
|
|
Degree of Bond Polarity |

Degree of unequal sharing of e⁻ between respective atoms. •Covalent: Equal share of electrons (homonuclear) •Polar covalent: unequal share e⁻. The EN atom has more e⁻ density (heteronuclear) •Ionic: transfer of electron from a low EN atom to a high EN to form a cation and anion |
|
|
Bonding and states of matter in covalent
|
Can be 3D solids but not required
This bonding is found with small molecules |
|
|
Bonding and states of matter in metallic/ionic
|
3D network of interactions
Solids or liquids but never as gas state |
|
|
Octet rule |
A general trend that atoms try to satisfy for stability. A filled valance orbital - 8 e⁻. |
|
|
Hypervalency
|
Central atoms which break the Octet rule of having 8 val. e⁻ around an atom, e.g. SF₆.
Period 3 and subsequent elements show deviation from the Octet rule, compared to period 2. As a result of low-lying unfolded d-orbitals which are able to accommodate the extra e⁻. |
|
|
Lewis Acid |
e⁻ pair acceptor- electron-deficient (electrophile). E.g. BF₃ |
|
|
Lewis Base |
e⁻ pair donator - electron rich (nucleophile). E.g. NH₃ |
|
|
Dative Covalent Bond |
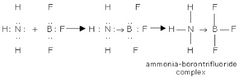
An e⁻ lone pair which is provided by 1 respective atom only. |
|
|
Working out Lewis Structure |
1.Calculate Σval. e⁻ 2.Arrange atoms in order of connectivity 3.Distribute in pairs (2 for each bond) 4.Assign double bonds/LP until each atom is satisfied. Note: •For cations subtract e⁻. •For anions add e⁻. |
|
|
Brønsted-Lowry Acid |
[H⁺] donor |
|
|
Brønsted-Lowry Base |
Accepts [H⁺] |
|
|
VSEPRT (Valence Shell Electron Pair Repulsion Theory)
|
1. Decide on central atom (most electro +VE).
2. Deduce Σval. e⁻ around the central atom inc. adjacent bonding e⁻. 3. Distribute Σval. e⁻ as BP / LP. 4. Identify geometry. Note: •BP and LP of e⁻ around centre atom are positioned as far apart as possible to reduce repulsion. •Double bonds count similar to single bonds. |
|
|
VSEPR - Multiple Bonds |
Dealing with molecules with double/triple Bonds (π bonds): •The π bonds occupy the same space as the σ e⁻. So double bonds count similar to single bonds. I.E. a double bond contains 2 e⁻ to count for.
|
|
|
VSEPR Example POCl₃ |
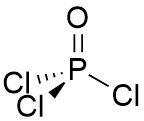
Central atom: P (grp. 15) •P: 5 val. e⁻ •Cl (x3): 3 val. e⁻ •O (2x1): 2 val. e⁻ (double bond) Σval. e⁻ = 10. 5 e⁻ pairs but 2 e⁻ in a π bond so 10 - 2 = 8 e⁻ for σ bonding. ∴ Tetrahedral arrangement |
|
|
VSEPRT - Ions |
•For anions add relevant e⁻ on the central atom based on the charge. •For cations subtract relevant e⁻ on the central atom based on the charge. |
|
|
VSEPR Example SO₄²⁻ |
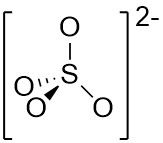
Central atom: S (grp. 16) •S: 6 val. e⁻ •O (2x4): 8 val. e⁻ •Add 2 from –VE charge Σval. e⁻ = 16 but 8 e⁻ in π bonds so 16 - 8 = 8 electrons for σ ∴ Tetrahedral arrangement |
|
|
LP Equatorial Vs. LP Axial? |
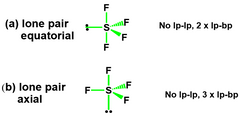
The more stable structure will have minimised e⁻ - e⁻ repulsion. •LP – LP > LP – BP > BP – BP. For most repulsion. Thus, (A) is preferred |
|
|
VSEPR Example 4 SF₄ Consideration: Think about the position of the e⁻ pair on the trigonal bipyramidal. Is it equatorial or axial site? |
Central Atom S ( grp.6) •S: 6 valence electrons •F (x4): 4 valence electrons Σval. e⁻ = 10, i.e. 5 e⁻ pairs
•4 bond pairs •1 lone pair ∴ Trigonal bipyramid |
|
|
Linear |
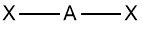
No. of e⁻ pairs: 2 No. of BP pairs: 2 No. of LP pairs: 0 θ = 180° |
|
|
VSEPR Example BeCl2 |
Central atom Be (group 2) •Be: 2 val. e⁻ •Cl (x2): 2 val. e⁻ Σval. e⁻ = 4 i.e. 2 pairs •2 bond pairs only ∴ shape is linear |
|
|
Trigonal planar |
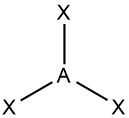
•No. of e⁻ pairs: 3 •No. of BP pairs: 3 •No. of LP pairs: 0 •θ =120° |
|
|
Tetrahedral |
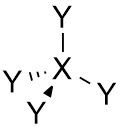
•No. of e⁻ pairs: 4 •No. of BP pairs: 4 •No. of LP pairs: 0 •θ =109.5° |
|
|
Trigonal bipryamidal |
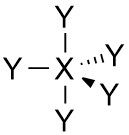
•No. of e⁻ pairs: 4 •No. of BP pairs: 5 •No. of LP pairs: 0 •θ = 90° (axial) and 120° (equatorial) |
|
|
VSEPR Example: PCl₅ |
Central atom P (grp. 15) •P - 5 val. e⁻ •Cl (x5) - 5 val. e⁻ Σval. e⁻ = 10, i.e. 5 pairs •5 BP - ∴ shape is trigonal bipyramidal |
|
|
Octahedral |
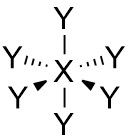
No. of e⁻ pairs: 6 No. of BP pairs: 6 No. of LP pairs: 0 θ = 90° |
|
|
Bent geometry |
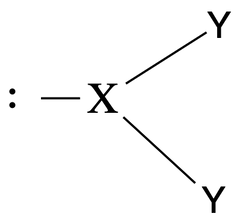
No. of e⁻ pairs: 3 No. of BP pairs: 2 No. of LP pairs: 3 θ = <120° |
|
|
Triggonal Pyramidal |
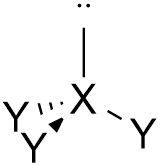
No. of e⁻ pairs: 4 No. of BP pairs: 3 No. of LP pairs: 1 θ = <109.5° |
|
|
VSEPR Example: NH₃ |
Central Atom N (grp. 15) •N - 5 valence electrons •3 x H - 3 valence electrons Σval. e⁻ = 8 i.e. 4pairs •3 bond pairs •1 lone pair ∴ arranged as a trigonal pyramidal (distorted tetrahedral). |
|
|
Bent (distorted tetrahedral) |
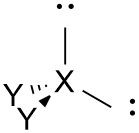
No. of e⁻ pairs: 4 No. of BP pairs: 2 No. of LP pairs: 2 θ = <109.5°
|
|
|
See-Saw |
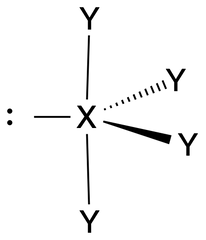
No. of e⁻ pairs: 5 No. of BP pairs: 4 No. of LP pairs: 1 θ = <120° (equatorial), 90° (axial). |
|
|
Square Pyramidal |
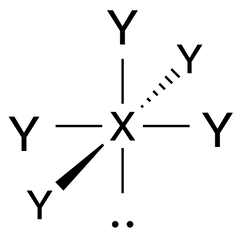
No. of e⁻ pairs: 6
No. of BP pairs: 5 No. of LP pairs: 1 θ = 90° |
|
|
T-Shaped |
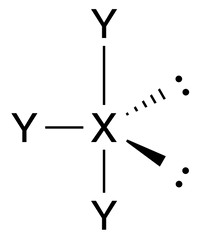
No. of e⁻ pairs: 5
No. of BP pairs: 3 No. of LP pairs: 2 θ = 120° (equatorial), 90° (axial) |
|
|
Square Planar |
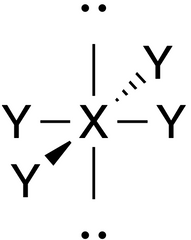
No. of e⁻ pairs: 6 No. of BP pairs: 4 No. of LP pairs: 2 θ = 90° |
|
|
Confronting Molecular Orbitals |
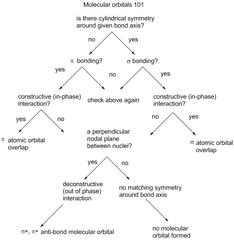
|
|
|
MO theory
|
MO theory allocates e to MO. Formed by AO with comparable E. and same symmetry with respect to the bond axis.
It can be summed up in 4 main points |
|
|
MO Principle 1: Σ No. MO = Σ No. AO contributed by the atoms that have combined. |
When atomic orbital “A” and “B” both bond together ---> x2 MO. E.G. take H₂, both AO is a 1s orbital. If 2 were to join there would be 2 MO (bonding and anti-bonding). |
|
|
MO Principle 2: In terms of E. bonding orbitals < parent orbitals. Whilst, anti-bonding orbital > than the parent orbital. |
Bonding MO are constructive (in-phase) interactions between AO in a molecule. e in bonding orbitals help stabilize the system Antibonding electrons formed by deconstructive (out-of-phase) combination of AO. These destabilized system. |
|
|
Principle 3: Electrons are assigned to orbitals of successively higher E. (Aufbau principle) |
Electrons fill bonding orbitals first since lower E than the anti bonding (Aufbau principle). No more than 2 electrons can go into each orbitals and are singularly filled before pairing ( Hund's rule). |
|
|
Principle 4 AO ---> MO most effectively when the AO is of similar E. |
MO likely to form when AO is of similar E. E.G. 1s AO combinining with another 1s AO. Forming a stable MO. |
|
|
Calculating BO |

Stability of a molecule can be determined via calculating BO. I.E: 0 - no bond 0.5 - half-bond 1 - 1 bond 2 - 2 bonds 3 - 3 bonds |

