![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
18 Cards in this Set
- Front
- Back
- 3rd side (hint)
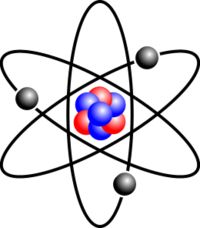
Atom
|
Smallest particle of an element
|
A water molecule has 3 atoms
|
|

Atomic mass
|
Average mass of atoms in an element
|
The atomic mass of Lithium is 6.941
|
|

Atomic Mass Unit
|
1/12 the mass of the mass of C12
|
The atomic mass is described by the atomic mass unit.
|
|
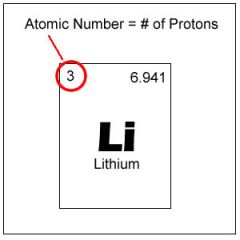
Atomic Number
|
Number of protons in the nucleus of an atom
|
The atomic number of Lithium is 3
|
|

Atomic Symbol
|
Tells mass number and number of protons
|
The atomic symbol of Carbon tells that the atomic mass is 12amu and there are 6 protons in every atom.
|
|
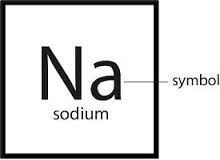
Chemical Symbol
|
1-3 letters representing an element
|
The chemical symbol for sodium is Na.
|
|
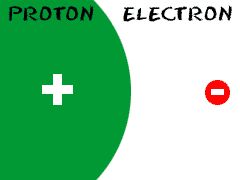
Electron
|
Particle of an atom with a negative charge
|
Carbon has 6 electrons.
|
|
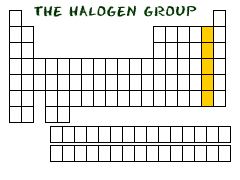
Group
|
Elements of a vertical column in the periodic table
|
The noble gases are a group
|
|
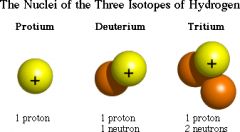
Isotope
|
One of two or more atoms having the same number of protons but different numbers of neutrons
|
Hydrogen has 3 isotopes
|
|
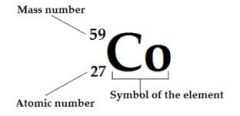
Mass Number
|
Total number of protons and neutrons in an atom
|
The mass number of carbon is 12
|
|

Metal
|
any of a class of elementary substances that are crystalline when solid and are characterized by opacity, ductility, conductivity, and a unique luster
|
Copper and gold are metals.
|
|

Metalloid
|
an element whose properties are intermediate between those of metals and solid nonmetals
|
Germanium is a metalloid.
|
|
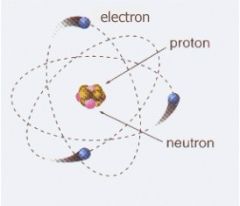
Neutron
|
Subatomic particle without an electric charge
|
Neutrons are in the nucleus.
|
|

Nonmetal
|
Substance without the same characteristics as a metal.
|
Nitrogen is a nonmetal.
|
|
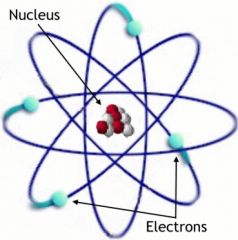
Nucleus
|
Center of the cell containing protons and neutrons. Makes up most the the cell's mass.
|
A nucleus has protons and neutrons.
|
|
Period
|
Name of a horizontal row on the periodic table.
|
Period 1 contains hydrogen and helium.
|
|
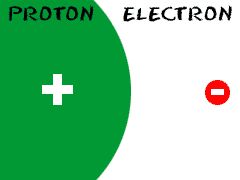
Proton
|
Positively charged subatomic particle located in the nucleus.
|
Protons are in the nucleus with neutrons.
|
|
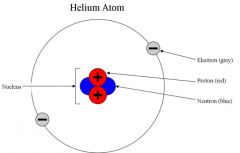
Subatomic Particle
|
a particle smaller than an atom
|
Protons and neutrons are subatomic particles
|

