![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
44 Cards in this Set
- Front
- Back
|
What are subatomic particles |
Protons Neutrons Electrons |
|
|
Protons |
Charge of +1 Mass of 1 amu Found in nucleus |
|
|
What is amu |
Atomic mass unit |
|
|
Electrons |
Charge of -1 Mass of 0 amu (1/1800) Found in the space outside a nucleus
|
|
|
Neutrons |
Charge of 0 Mass of 1 amu Found in nucleus |
|
|
What is the atomic number |
The number of protons found in the nucleus of an atom (Also electrons) |
|
|
Isotopes |
Atoms of the same element, with the same number of protons, but different numbers of neutrons |
|
|
Atomic mass |
Protons + Neutron |
|
|
Ions |
When an atom loses or gains an electron |
|
|
Radioactivity |
When substances spontaneously wmit radiation |
|
|
Nuclear reactions |
Reactions that involve a change in the atom's nucleus The atom the one element can change into the atom of another element |
|
|
Radiation |
The Ray's and particles emitted by the radioactive material |
|
|
radioactive decay |
unstable nuclei lose energy by emitting radiation in a spontaneous process (doesn't require energy) |
|
|
alpha radiation |
radiation that is positive |
|
|
alpha particles |
contains 2 protons and 2 neutrons, 2+ charge |
|
|
nuclear equation |
show the atomic number and mass number of particles involved |
|
|
Beta radiation |
Radiation that deflects towards the positively charged plate |
|
|
Beta particles |
Radiation that consists of fast-moving electrons called beta radiation Electron with -1 charge |
|
|
Gamma radiation |
Gamma rays are high-energy radiation that possess no mass No electrical charge not deflected by electric or magnetic fields |
|
|
Nuclear stability |
An atom's stability is determined by its ratio of neutrons to protons |
|
|
Electromagnetic radiation |
Form of energy that exhibits wavelike Behavior as a travels through space |
|
|
Examples of electromagnetic radiation |
Visible light, microwaves, x-rays, radio, television waves |
|
|
Wavelength |
Shortest distance between equivalent points on a continuous wave |
|
|
Units of wavelength |
Meters, centimeters, nanometers |
|
|
Frequency |
The number of waves that pass a given point per second |
|
|
Amplitude |
The waves height from the original to Crest or from origin to a trough |
|
|
Electromagnetic spectrum |
Encompasses all forms of electromagnetic radiation with the only differences in the types of radiation being their frequencies and wavelengths |
|
|
Quantum theory |
The description of the properties of atoms using wave properties Based on the idea that we can only predict the probability of finding an electron in a particular position |
|

Who's model is this and what is it called |
Dalton's model Marble model |
|
|
What what Dalton's theory (marble model) |
All matter is made of indivisible and indestructible atoms Atoms of a given element are identical in their properties Atoms of different elements have different properties Law of multiple proportions |
|
|
Law of multiple proportions |
Atoms of different elements combine in simple whole number ratios to form compounds |
|
|
What was Dalton's conclusion (marble model) |
Law of conservation of mass |
|
|
Law of conservation of mass |
Atoms cannot be subdivided, created, or destroyed when they are combined, separated or arranged in chemical reactions |
|

Who's model is this and what is it called? |
J. J. Thompson's model Plum Pudding model |
|
|
What was J.j. Thompson's conclusion |
Negatively charged electrons embedded and ball positive positive charge |
|
|
Robert a millikan |
Used Thompson's work to determine the exact charge and mass of an electron |
|
|
Ernst Rutherford experment? |
Gold foil experienment |
|
|
Ernst Rutherford theory |
The positive particles would deflect as they Struck the positive spheres of the plum pudding model atom |
|
|
Ernst Rutherford conclusion |
Most of the atom is empty space
Adams have a solid core called the nucleus
Nucleus are positively charged
They measure the approximate size of the nucleus |
|
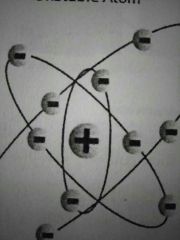
|
Rutherford model |
|

|
Bohr model |
|
|
Neil Bohr |
Electrons would be found only in specific energy levels, similar to the rungs on a ladder Energy levels closer to the nucleus have a lower energy from those further away The difference in energy between anyone level and the next called a Quantum |
|
|
atom |
basic unit of matter |
|
|
elements |
a substance in which all the atoms are exactly the same |

