![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
28 Cards in this Set
- Front
- Back
|
What are the structure and function of the major parts of the digestive system?
|
- The Oesophagus; made up of thick muscular wall carries food from the mouth to the stomach
- The Stomach; muscular sac with an inner layer that produces enzymes. It's role is to store and digest food, especially proteins. It also produces mucus to prevent itself being digested by it's own enzymes. - The Small Intestine; a long muscular tube, food is further digested there by enzymes that are produced by it's walls and by glands that pour their secretions into it. Folded into villi and microvilli to adapt it for absorbing the products of digestion into bloodstream. - The Large Intestine; absorbs water - The Rectum; the final section of the intestines where the faeces are stored. - The Salivary Glands; situated near mouth, pass it's secretions via a duct into mouth. - The Pancreas- large gland situated below the stomach and it produces pancreatic juice. |
|
|
How does the digestive system break down food both physically and chemically?
|
Physical- large bits of food are broken down into smaller ones e.g. teeth and stomach muscles.
Chemical- breaks down large insoluble molecules into smaller soluble ones. It is carried out by enzymes. |
|
|
What is the role of enzymes in digestion?
|
Enzymes function by hydrolysis- splitting up of molecules by adding water to the chemical bonds that hold them.
Enzymes are specific; - Carbohydrases break down carbohydrates- to monosaccharides. - Lipases break down lipids into glycerol and fatty acids. - Proteases break down proteins to amino acids. |
|
|
How are large molecules like carbohydrates constructed?
|
They are made up of a chain of individual molecules . Each of the individual molecules that make up this chain are called monomers. The carbon atoms of these monomers join to form a longer chain called polymers.
In carbohydrates the basic monomer unit is a sugar, otherwise known as a saccharide. |
|
|
What is the structure of a monosaccharide?
|
Monosaccharides are sweet-tasting, soluble substances that have the general formula (CH2O)n where n can be any number from 3 to 7.
Glucose is a monosaccharide with a formula C6H12O6. |
|
|
How would you carry out the Benedict's test for reducing?
|
All monosaccharides and some disaccharide are reducing sugars. The test for a reducing sugar is known as the Benedict's Test.
Benedict's Test: 1) Add the food sample to be tested, if not liquid then grind it up in water. 2) Add an equal volume of Benedict's reagent. 3) Heat the mixture in a gently boiling water bath for 5 minutes. If a reducing sugar is present he solution turn orange-brown and if not it remains blue. |
|
|
How are monosaccharides linked together to form disaccharides?
|
When monosaccharides join a molecule of water is released and a reaction is therefore called condensation reaction and the bond that is formed is called a glycosidic bond.
Glucose and Glucose make maltose. Glucose and Fructose make sucrose. Glucose and Galactose make lactose. |
|
|
How are alpha-glucose molecules linked to form starch?
|
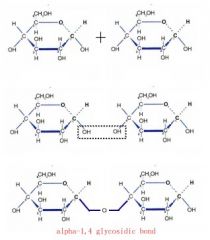
This is how a glycosidic bond is formed and in order to form starch many glucose molecules have to join in this way.
|
|
|
What is the test for non-reducing sugars?
|
In order for a non-reducing sugar to be detected it must first be broken down into it's monosaccharide components by hydrolysis. So to test for a non-reducing sugar we di a modified Benedict's Test:
1) Add the sample to be tested if not liquid then grind it up in water. 2) Add equal volume of dilute hydrochloric acid and place the test tube in a gently boiling water bath for 5 minutes. 3) Add some hydrogencarbonate to neutralise the acid. 4) Add Benedict's reagent and heat in a gently boiling water bath for 5 minutes. 5) If a non-reducing sugar is present the sample will turn orange-brown if not it will remain blue. |
|
|
What is the test for starch?
|
1) Place the sample to be tested into a test tube.
2) Add two drops of iodine solution and shake or stir. The presence of starch is indicated by a blue-black colour. |
|
|
How does salivary amylase act in mouth to hydrolyse starch?
|
When saliva enters the mouth it is thoroughly mixed with the food during chewing. The saliva contains salivary amylase this starts hydrolysing any starch in the food to maltose. It hydrolyses the starch by adding a water molecule to the bonds to break them.
|
|
|
How is starch digestion completed in the small intestine?
|
In the intestine the food is mixed with a secretion called pancreatic juice. The pancreatic juice contain pancreatic amylase and this continues the hydrolysis of any remaining starch to maltose.
|
|
|
How are disaccharides digested?
|
Sucrose and Lactose are the other two main disaccharides that need to be broken down. Sucrose is broken down by enzyme called sucrose and lactose is broken down by enzyme called lactase. Both enzymes are produced by the lining of the small intestine.
|
|
|
What is lactose intolerance?
|
Some people do not produce sufficient lactase to digest all the lactose they consume . When the undigested lactose reaches the large intestine, microorganisms break it down, giving rise to a large volume of gas. This may result in bloating, nausea, diarrhoea and cramps. Some people with this condition cannot consume milk or milk products at all whereas other can consume only a little.
|
|
|
How are amino acids linked to form polypeptides- the primary structure of proteins?
|
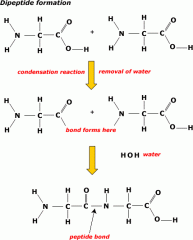
Amino acids are the monomers and they combine to form a dipeptide and polypeptide by the condensation reaction. The joining of two amino acids forms a peptide bond.
The sequence of amino acids in a polypeptide chain forms the primary structure of any protein. |
|
|
How are polypeptides arranged to from the secondary and then the tertiary structure of a protein?
|
Secondary Structure- Weak hydrogen bond are formed rapidly and the polypeptide chain twist into a 3D structure called alpha-helix or beta-pleated sheet.
Tertiary Structure- The protein is twisted and folded even more. The new 3D shape is held in place by disulphide bonds (fairly strong), ionic bonds (weaker but still strong and broken by changes in pH) and hydrogen bonds (weak and numerous). |
|
|
How is a quaternary structure of a protein formed?
|
Quaternary structure is two or more polypeptide chains linked together; there may also be a non-protein groups attached.
|
|
|
How are proteins identified (test for proteins)?
|
Biuret test detects peptide links so it is the most reliable protein test.
Place a sample of the solution to be tested in a test tube and add an equal volume of a Biuret reagent. A purple coloration indicates the presence of peptide bonds hence the presence of a protein. If no protein is present, the solution remains blue. |
|
|
How do enzymes speed up the chemical reactions?
|
They lower the activation energy of the reaction and do not get used up during a reaction.
|
|
|
How does the structure of enzyme molecules relate to their function?
|
Enzymes are globular protein, they have a specific 3D shape which is a result of their primary structure. The enzyme molecule is large overall however only a small region of it is functional- it's active site. Enzymes work on substrates. The specific 3D shape relates to it's function as it can only work on one substrate so it has an active site complementary just to one type of a substrate.
|
|
|
What is a lock and key model of enzyme action?
|
The enzyme works in the same way as a key operates a lock; each key has a specific shape that fits and operates only a single lock. In a similar way a substrate will only fit the active site of one particular enzyme. This model is supported by the observation that enzymes are specific in the reactions that they catalyse. The shape of the substrate (key) exactly fits the active site of the enzyme (lock). A limitation is that enzyme is not a rigid structure.
|
|
|
What is the induced-fir model of enzyme action?
|
This model proposes that the enzyme changes it's shape slightly to fit the profile of the substrate. So the enzyme is flexible and can mould itself around the substrate like a glove moulds itself to the shape of the hand. The enzyme has it's general shape but alters in the presence of a substrate. As it alters it's shape the enzyme puts a strain on the substrate molecule, this strain distorts a particular bond and lowers the activation energy. This model is a better explanation as it explains how other molecules can affect enzyme activity and how the activation energy is lowered.
|
|
|
How is the rate of an enzyme-controlled reaction measured?
|
To measure the progress of an enzyme- catalysed reaction we usually measure it's time-course (how long it takes for a particular event to run it's course). This can be measure in two ways:
- The formation of products of the reaction - The disappearance of the substrate |
|
|
How does temperature affect the rate of an enzyme-controlled reaction?
|

|
|
|
How does pH affect the rate of an enzyme-controlled reaction?
|

|
|
|
How does substrate concentration affect the rate of reaction?
|

|
|
|
How does competitive inhibitors and non-competitive inhibitors affect the active site?
|
Competitive inhibitors have a molecular shape similar to that of the substrate and this allows them to occupy the active site of an enzyme.
Non-competitive inhibitors attach themselves to the enzyme at a site which is not the active site. Upon attaching to the enzyme, the inhibitor alters the shape of the enzyme's active site in such a way that substrate molecules can no longer occupy it and the enzyme cannot function. |
|
|
What does enzyme inhibition graph look like?
|
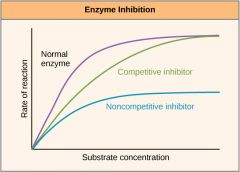
|

