![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
6 Cards in this Set
- Front
- Back
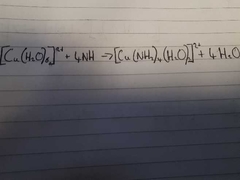
What is the colour change for this reaction? Explain the reaction. |
Pale Blue to Dark Blue A pale blue prcipitate of Cu(OH)2 is formed. The Cu(OH)2 percipitate then dissolves in excess ammonia to form a dark Blue Solution. |
|
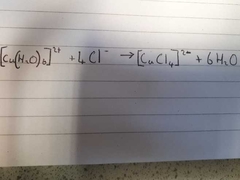
What is the colour change for this reaction? |
Pale Blue Octahedral to Yellow Tetrahedral |
|

What is the colour change for this reaction? |
Green percipitate to dark green solution |
|

What is the colour of this 3D structure? |
Blue |
|
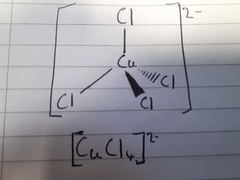
What is the colour of this 3D structure? |
Yellow |
|
|
Explain how to carry out a redox titration involving Manganate VII (MnO4-). |
Add a known volume of Manganate ions /MnO4- (Purple)in aqueous potassium manganate to a known volume of reducing agents i.e. Fe2+. Gradually add untill all the reducing agent is used up causing a colour change i.e. colourless to purple. This is the end point of the reaction. |

