![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
77 Cards in this Set
- Front
- Back
|
How is iron (Fe) used in the body? |
Hemeproteins: Hb, Mb, cytochromes, catalase
Non-hemproteins: FeS proteins, flavoproteins |
|
|
How much iron is normally in the body? |
4 g |
|
|
Where is most of the body's iron located? |
In Hb (2.5 g) |
|
|
What is a newborn's dietary iron requirement? |
ZERO for the first several months
Why:
In newborns, RBC destruction >> erythropoiesis; Fe is salvaged and stored; no Fe lost in stools
|
|
|
Is there iron in milk? |
Yes, but it is incorporated in lactoferrin (a protein), making it unavailable |
|
|
What is the hypothesized function of lactoferrin? |
It may be an Fe scavenger that retards bacterial growth |
|
|
What is an adult's RDA of iron? |
10x normal loss (only about 10% of Fe is absorbed in the gut)
Males: 10 mg/day (about 1 mg/day absorbed)
Females: 18 mg/day (about 1 mg/day absorbed) |
|
|
When is a male's greatest need for iron? |
During puberty |
|
|
When is a female's greatest need for iron? |
Menstruation: lose about 100 mg Fe (~100 mL of blood lost)
Pregnancy: 300 mg Fe → fetus (3rd trimester)
Lactation: production of lactoferrin |
|
|
Which oxidation sate of iron is found in nature? |
Fe³⁺ (ferric)
Useless in the human body |
|
|
What must happen before dietary iron can be absorbed into the mucosal cells of the intestine? |
It must be reduced to Fe²⁺ (ferrous) by vitamin C |
|
|
What happens to iron once it is absorbed into intestinal mucosal cells? |
It is either stored by ferritin (as Fe³⁺) or transported into the bloodstream |
|
|
How is iron transported from mucosal cells into the blood stream?
|
The iron-sensing complex (HFE, β2-microglobulin, other proteins) located on the serosal side of intestinal cells
This is where regulation of iron metabolism occurs |
|
|
How is iron transported in the blood? |
By the carrier protein transferrin (Fe³⁺) |
|
|
How is iron oxidized (Fe²⁺→Fe³⁺) for transport by transferrin? |
By the copper-containing enzyme Ceruloplasmin (ferroxidase) |
|
|
How is transferrin internalized by the tissues? |
Via transferrin receptor-mediated endocytosis (in clathrin-coated pits) |
|
|
What is the fate of iron in most tissues? |
It is stored by ferritin or incorporated into many enzymes and cytochromes |
|
|
Where does most of the dietary iron go? |
To bone, where it is incorporated into heme for RBC's |
|
|
How often are RBC's turned over?
|
Every 120 days
|
|
|
What happens to iron when RBC's are destroyed? |
It is transferred by transferrin from the cells of the reticuloendothelial system back to bone for reincorporation into RBC's |
|
|
Iron Deficiency Anemia |
RBC appearance:
Microcytic Hypochromic
Much like vitamin B₆ deficiency (pyridoxal-phosphate required for ALA synthase) |
|
|
How can a physician distinguish iron deficiency from vitamin B₆ deficiency? |
In B₆ deficiency: sideroblastic anemia (due to iron in mitochondria)
In iron deficiency: Zn²⁺-protoporphyrin (fluorescent RBC's) |
|
|
Ferritin (Function, Location) |
Function: iron storage protein
Location: intestine, liver, spleen, and bone marrow |
|
|
Apoferritin |
Ferritin with no iron |
|
|
How many iron atoms can ferritin bind? |
4,300 Fe/protein |
|
|
What does a high blood ferritin level indicate? |
Iron overload |
|
|
Transferrin (Function, Location) |
Function: iron transport glycoprotein
Location: blood |
|
|
How many iron atoms can tranferrin bind at once? |
Up to 2 Fe³⁺/protein |
|
|
What is the normal iron-binding capacity of transferrin? |
1/3 saturated |
|
|
What is the normal plasma [iron]? |
120 µg/dL |
|
|
Why must iron be transported in the bloodstream and stored in complex with a protein?
|
Iron forms insoluble salts with anions
|
|
|
Total Iron-Binding Capacity (TIBC) |
Serum blood test for Fe
High TIBC = iron deficiency Low TIBC = iron overload |
|
|
Why does the body tightly regulate iron absorption? |
There is no biochemical excretory pathway for iron |
|
|
Hemosiderin |
An aggregate of ferritin laden with iron (up to 37% iron)
Accumulates during iron overload
Essentially useless, and can precipitate in the liver, causing damage |
|
|
Hemochromatosis |
Inherited, autosomal recessive disease of iron metabolism (fairly common in Northern Europe)
Caused by a missense mutation in HFE gene (part of iron-sensing complex)
Causes absorption of 2-3 mg/day of iron (compared to 1 md/day normally)
Over 20-30 years, results in levels of 20-30 g of iron in the body (normal = 4 g)
Hemosiderin deposits cause:
Liver cirrhosis Diabetes Dermatitis Arthritis
Also characterized by bronze skin |
|
|
Treatments for Hemochromatosis |
Phlebotomy or Deferoxamine (Fe chelator) |
|
|
Secondary Hemochromatosis |
Elevated iron due to use of iron supplements |
|
|
Why is it important to have a TIBC performed before taking iron supplements? |
The initial symptoms of both iron deficiency and overload are the same: tired, lethargic |
|
|
Bulk Elements |
>100 mg/day
|
|
|
Properties of Minerals |
Most cation minerals (excepts Na⁺ and K⁺) form insoluble salts with phosphates, oxalates, and phylates → not readily absorbed
Most require specific proteins for absorption, transport, and storage |
|
|
Phylate |
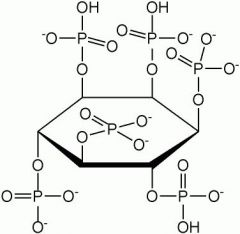
Found in wheat
Broken down by yeast |
|
|
Excretion of Minerals |
(Most) Kidney → urine
Liver → bile → feces |
|
|
How are body [mineral] regulated? |
By absorption and secretion |
|
|
Cause of Mineral Deficiencies |
Most are secondary to malabsorption |
|
|
Causes of Mineral Toxicity |
Excessive intake
Control of absorption is impaired
Renal problems |
|
|
Treatment for mineral toxicity |
Chelation |
|
|
Cobalt (Function) |
Component of vitamin B₁₂
Co + Flora → vitamin B₁₂ |
|
|
Copper (Function, Sources) |
Function: cytochrome oxidase, lysyl oxidase (collagen synthesis), superoxide dismutase
Sources: meats, nuts, cereals, raisins, legumes
About 100 mg in the body |
|
|
Where is copper stored in the body? |
Intestine and liver |
|
|
How is copper stored? |
By the storage protein metallothionein |
|
|
How is copper transported in the bloodstream? |
Bound to serum albumin |
|
|
What is copper's fate in the liver? |
Storage with metallothionein
Incorporation into ceruloplasmin
Excretion in bile → feces |
|
|
Ceruloplasmin (Ferroxidase) |
Bright blue enzyme (due to 6-8 Cu atoms)
Made in the liver
Oxidizes Fe²⁺ to Fe³⁺ |
|
|
Menkes Disease |
Defect in Cu-ATPase (ATP7) in serosal side of the intestine
↓ Cu in the blood ↓ Cu in the liver
Symptoms:
Kinky hair Hypopigmentation Neurological problems
Treatment: IV copper |
|
|
Wilson's Disease |
Defect in Cu-ATPase (ATP7B) in liver exit into bile
↓ Cu in the blood (liver can't make ceruloplasmin) ↑ Cu in the liver (cirrhosis)
Symptoms:
Kayser-Fleischer ring (Cu in Descemet's membrane of eye)
Treatment:
Penicillamine or Trientine Metallothionein inducing drugs (Galzin) Zinc (blocks intestinal Cu absorption) |
|
|
Why can zinc therapy be used to treat Wilson's disease? |
Zinc uses the same intestinal transporter as Copper, but it uses a different excretion pathway |
|
|
Chromium (Function, Sources) |
Function: enhances insulin binding to its receptor
Sources: grains, cereals, stainless steel cookwares |
|
|
Chromium Deficiency |
Increase in glucose intolerance |
|
|
Fluorine (Function, Sources) |
Function: protects against carries
Sources: water (found naturally and added for protection against carries) |
|
|
How does fluorine protect against carries? |
The presence of fluorine promotes the formation of fluoroapatite, which is resistant to acids, over hydroxyapatite
Ca₁₀(PO₄)₆(OH)₂ → Ca₁₀(PO)₄F₂
It is unknown whether fluorine has this effect in adults |
|
|
Iodine (Function, Sources) |
Function: synthesis of thyroid hormones (regulate BMR, growth, and development of children) from thyroglobulin
Sources: saltwater fish
50 mg in the body (most in thyroid) |
|
|
Iodine Deficiency |
Goiter (enlarged thyroid) |
|
|
Manganese (Function) |
Cofactor for enzymes involved in protein glycosylation |
|
|
Manganese Deficiency |
Testicular degeneration |
|
|
Molybdenum (Function) |
Xanthine oxidase (produces uric acid) and other oxidases |
|
|
Selenium (Function) |
Component of glutathione peroxidase (selenocysteine)
Like S, Se serves as an anti-oxidant
|
|
|
Selenocysteine |
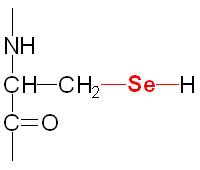
Component of glutathione peroxidase |
|
|
Selenium Toxicity |
Garlicky breath, exhalation of selenoxide |
|
|
Selenoxide |
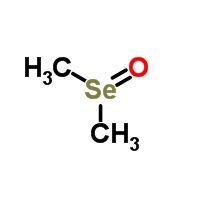
|
|
|
Zinc (Function, Source) |
Function: component of at least 2 dozen enzymes
Source: meats |
|
|
How is zinc absorbed? |
It uses the same intestinal transporter as Cu |
|
|
Where is zinc stored? |
Same as Cu, in the intestine and liver |
|
|
How is zinc stored? |
Same as Cu, bound to matallothionein |
|
|
How is zinc transported in the blood? |
Same as Cu, bound to serum albumin |
|
|
What is the fate of Zinc in the liver? |
Stored with metallothionein
Incorporated into many enzymes
Excreted in bile → feces |
|
|
How is most zinc excreted? |
In pancreatic juice (can be used to diagnosis pancreatitis) |
|
|
Causes of Zinc Deficiency |
Acradermatitis enteropathica (rare genetic absorption problem)
Malabsorption
Sick cell anemia → ↑ Zn in urine
Phytic acid → eating unleavened bread |

