![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
83 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
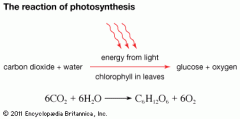
Explain in brief the reaction of CO2 and water being converted into glucose and oxygen.
|
–Carbon is "fixed from CO2
–Water is split. H is used to help make glucose, but O is excreted as waste. –light energy is used to form glucose. Can be stored as starch or cellulose. |
|
|
|
What is one use of energy in photosynthesis? Explain.
|
–Photolysis––the splitting of water molecules
–light dependent reaction that splits water, making H+ ions and ATP. –This produces Oxygen as a waste product |
|
|
|
What type of reaction leads to the fixation of CO2 in order to make glucose? How does it do this?
|
Light independent reaction. Uses ATP and H+ ions.
|
|
|
|
How does can carbohydrates act as energy storage in plants?
|
larger molecules tend to contain more bonds than smaller ones. Therefore more ATP is required to build bonds and generate larger molecules. Consequently large molecules can act as energy stores.
|
|
|
|
When a reaction is endothermic, what does this mean?
|
The reaction absorbs energy from their surroundings.
|
|
|
|
What is the process through which monosaccharides are combined to make carbohydrates?
|
Condensation. Molecules of glucose form glycosidic bonds to create a polysaccharide and water.
|
|
|
|
High frequency radiation is ________ _________ and ___________ to living organisms because they ___________ cell and DNA damage.
|
high energy, harmful, encourage
|
|
|
|
What is the purpose of the pigments in photosynthetic organisms?
|
Chlorophyll and other pigments are used to absorb useful wavelengths of light that contain energy for photolysis in light dependent reactions.
|
|
|
|
Why does a leaf look green?
|
Chlorophyll is the main photosynthetic pigment. It absorbs Blue and Red light, but reflects green light. We only see the colours of light that matter reflects, so we see leaves as being green.
|
|
|
|
What is the action spectrum?
|
A spectrum showing the rate of photosynthesis for all wavelengths of light as a % of the maximum possible rate.
|

|
|
|
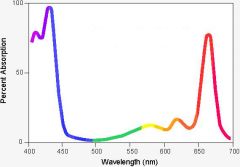
What is the absorption spectrum?
|
A spectrum that shows the absorbency of light by photosynthetic pigments (chlorophyll in most graphs) for all the wavelengths of light as a % of maximum absorption.
|
|
|
|
How do we know that chlorophyll is the most important of the photosynthetic pigments?
|
The absorption spectrum for chlorophyll and the action spectrum overlap each other.
|
|
|
|
Which has higher energy, red or blue light? Which is absorbed better, red or blue light?
|
Blue.
Red. |
|
|
|
Under what circumstances would plant use "accessory pigments"?
|
–underwater
–low light conditions to take advantage of green wavelengths –cold weather when chlorophyll break down but accessory pigments don't |
|
|
|
What factors effect the rate of photosynthesis?
|
light intensity
CO2 concentration Temperature |
|
|
|
How does CO2 effect the rate of photosynthesis?
|
–CO2 is a substrate for the metabolic pathway.
–when CO2 conc. increases the rate of photosynthesis increases –It is the limiting factor at low concentrations –There comes a point where another factor is limiting photosynthesis therefore increase of the gas does not increase the rate of photosynthesis, forming a plateau |
|
|
|
How does temperature effect the rate of photosythesis?
|
–Increase in temperature gives molecules more kinetic energy causing substrates to collide more frequently.
–At the optimum temperature, enzymes have begun to denature, causing the rate of photosynthesis to reach a peal –after the optimum temperature enzymes denature rapidly causing a fast decrease in the rate of photosynthesis |
|
|
|
How can you measure the rate of photosynthesis?
|
Measure CO2 uptake: place plant in closed, water filled space. CO2 reacts with water, increasing acidity. Measure acidity.
Oxygen production: In water, count bubbles, place in inverted measuring cylinder, use of oxygen probes, gas syringe. Change in biomass: Glucose production can be measured by change in plant's dry biomass. starch can be identified using iodine solution and colorimeter |
|
|
|
What are Photosystem II and Photosystem I? What is their job? |
Photosystem II and Photosystem I are protein complexes found in the thylakoid membrane. These proteins are used in the ETC to absorb light energy in order to excite electrons, and to split water molecules (photolysis) to free hydrogen ions (only PsII). These processes provide the energy and key ingredients required to produce glucose. |
|
|
|
What are the parts of a chloroplast? |
Chloroplast envelope--inner and outer membrane surrounding chloroplast. Stroma--site of light independent reactions (Calvin cycle) like the matrix
Thylakoid membranes + thylakoid stack--thylakoid membranes hold together grana (singular granum). Grana are made up of thylakoid disks |
|
|
|
What processes occur in Light Dependent Reactions? What goes in and what comes out? |
Photolysis and photophosphorylation In: H2O, NADP+, ADP Out: O2, NADPH, ATP |
|
|
|
What processes occur in LIght Independent reactions? What goes in and what comes out? |
Carbon fixation, Calvin Cycle, Carbohydrate synthesis In: CO2, NADPH, ATP Out: glucose phosphate, NADP+, ADP |
|
|
|
How do light dependent reactions begin (hint: photoactivation)? |
-Light enters the chloroplast and raises the energy level of electrons in the chlorophyll of PsII (photoactivation). -photoactivated electrons are passed along the membrane by electron carriers. They activate proton pumps. |
|
|
|
In the ETC, how is the hydrogen ion gradient created? (hint: photolysis) |
-PsII splits water molecules into 2 hydrogen ions and 1/2 O2. -proton pumps (which have been activated by the photoactivated electrons, pump more H+ into the thylakoid space. -the 2 electrons created replace the electrons that left PsII from photoactivation. |
|
|
|
How is ATP created in the ETC of photosynthesis? |
-H+ spin ATP synthase by flowing through it (chemiosmosis). -This is called non-cylic phosphorylation -ADP + Pi ---> ATP |
|
|
|
How is the concentration gradient in photosynthesis ETC maintained? |
-electrons in photosystem I are activated by light -they are received by ferredoxin and NADP+ reductase reduces NADP+ to NADPH with an H+ ion. -NADPH is carried to the light independent reactions. |
|
|
|
From left to right, what proteins would you draw in the thylakoid membrane while making a diagram of light-dependent reactions? |
PsII, PQ, cytochrome complex, PsI, ferredoxin, NADP+ reductase, ATP synthase. |
|
|
|
Why are light dependent reactions necessary for light independent reactions? |
-Light independent reactions use NADPH and ATP -NADPH and ATP are produced in light dependent reactions |
|
|
|
What is the first step of the Calvin Cycle? |
Carboxylation: -Ribulose Bisphosphate (RuBP) (5C) is carboxylated with CO2 and rubisco -the 6C product immediately spits into 2 glycerate 3 phosphate (G3P) |
|
|
|
What is the second step of the Calvin Cycle? |
Reduction: -G3P is reduced to triose phospate (TP) -this happens due to the oxidation of 2ATP to 2ADP and 2NADPH to 2 NADP+ |
|
|
|
What is the (pseudo) third step of the Calvin Cycle? |
Glucose synthesis: -1/6 of the triose phosphate molecules are linked to form glucose phosphate which turns into starch by condensation |
|
|
|
What is the last step of the Calvin Cycle? |
Regeneration: -5/6 of the TP molecules are used to regenerate RuBP -this is done by oxidizing ATP to ADP |
|
|
|
How does the chloroplast structure relate to its function? |
-Palisade cells--found in top surface of leaves, contain high density of chloroplasts to enable efficient absorption of light -grana--stacked thylakoid disks provide a large surface area for light absorption -chlorophyll and other pigments are grouped together to form photosystems embedded in the membrane with electron carriers -folds allow these to be close together -thylakoid spaces--small volume allows rapid accumulation of H+ -stroma--contains rubisco for carboxylation |
|
|
|
Compare and contrast chloroplasts and mitochondria |
chloroplast vs. mitochondria chloroplast envelop vs outer mitochondrial membrane--both compartmentalize organelle thylakoid membrane vs inner mitochondrial membrane--both have ETC and make ATP in the same way stacked membranes vs invaginated membranes--maximize surface area for reactions low intermembrane spaces--rapid H+ conc. stroma vs. matrix--fluid for cyclic reactions |
|
|
|
What does glycolysis do?
|
–Glycolysis splits glucose into two pyruvate molecules.
–uses two ATP to create 4 ATP –convert |
|
|
|
What does Glycolysis do?
|
–produces net 2 ATP (uses 2, produces 4)
–reduction reaction of NAD+ to NADH –splits glucose into 2 pyruvate |
|
|
|
Where does glycolysis occur?
|
In the cytosol (or cytoplasm)
|
|
|
|
What occurs in oxidative phosphorylation?
|
–NADH gets oxidized to NAD+ (losing electrons)
–This releases a lot of energy –Oxygen accepts these electrons and is turned to water (is reduced) –this happens in steps where energy is released when it is accepted enzymes which pump hydrogen protons across a membrane. |
|
|
|
How is ATP produced in oxidative phosphorylation?
|
–Electrons go down a gradient in the phospholipid bilayer when proteins in the membrane accept electrons from the oxidation of NADH to NAD+
–creates a proton gradient, more protons on the intermembrane space side. –Then they flow down a protein called ATP synthase, turning a rotor, and causing the creation of ATP. –ADP plus a phosphate group makes ATP |
|
|
|
What are the steps of cellular respiration? Where does each step occur?
|
Glycolysis in the cytoplasm
Link Reaction in the matrix Kreb's Cycle in the matrix Oxidative Phosphorylation (electron transport chian + chemiosmosis) in the matrix, inner membrane, and intermembrane space |
|
|
|
How much NADH is produced by the link reaction? How much ATP?
|
2 and 0
|
|
|
|
How much NADH is produced by glycolysis? how much ATP?
|
2 and 2
|
|
|
|
how many times is NAD+ reduced to NADH in the citric acid cycle?
|
3 times
|
|
|
|
How much NADH is produced in the Kreb's Cycle? How much ATP?
|
6 and 2
|
|
|
|
How does cell respiration start?
|
A Glucose (hexose) molecule is broken into two pyruvates in glycolysis.
|
|
|
|
What is a reduction reaction?
|
A reaction where electrons are gained, or oxygen is removed, or hydrogen is gained
|
|
|
|
What is an oxidation reaction?
|
A reaction where electrons are lost or oxygen is added, or hydrogen is lost.
|
|
|
|
What is the most common hydrogen carrier and what is its reduced and oxidized form?
|
NAD+, Nicotinamide Adenine Dinucleotide
If it goes through a reduction reaction, NADH is produced. If NADH is oxidized, NAD+ is produced |
|
|
|
What is the less frequent hydrogen carrier and what is its reduced and oxidized form?
|
FAD, Flavin Adenine Dinucleotide
If it goes through a reduction reaction, FADH2 is produced. If FADH2 is oxidized, FAD is produced |
|
|
|
What is an electron/hydrogen carrier?
|
A molecule that is capable of accepting electrons/hydrogen ions from another molecule (electron donor) and then transport these electrons to donate to another molecule during ETC.
E.g. NADH and FADH2 are co–enzymes that source electrons (and H+) in ETC Think of them as tickets for creating ATP |
|
|
|
Describe the first step of Glycolysis.
|
Substrate level phosphorylation:
–Glucose is phosphorylated to create Fructose 1,6 bisphosphate. –It is a six carbon molecule with a phosphate group on carbon 1 and 6 (making it unstable) –this requires the investment of 2 ATP. ATP is oxidized to give the energy to bond, producing 2 ADP + Pi |
|
|
|
Describe the second step of glycolysis.
|
Lysis:
–The Fructose 1,6 bisphosphate splits into two 3 carbon molecules called: –glyeralaldehyde 3 phosphate/PGAL/triose phosphate (TP) |
|
|
|
Describe the third step of glycolysis.
|
Oxidation:
–Another phosphate group is added to the PGAL –Oxidation removes hydrogen, which is used to reduce NAD+ to NADH –2 NADH are produced |
|
|
|
Describe the fourth step of glycolysis.
|
ATP formation:
–Through the reduction of 2 ADP + Pi to 2 ATP on each 3 C molecule, 4 ATP total are produced –As well, a 3C molecule called a pyruvate is formed. |
|
|
|
What is the result of glycolysis?
|
2 NADHs
2 net ATP (4 gross ATP) 2 3C pyruvates |
|
|
|
Why are phosphate groups added to molecules in glycolysis?
|
Phosphorylation, (the addition of phosphorous to a hydrocarbon or ADP molecule), makes a molecule less stable. This allows reactions to proceed faster without needing to use energy.
|
|
|
|
What occurs in the link reaction?
|
–A pyruvate binds with Coenzyme A through the decarboxylation and oxidation of of the pyruvate.
–This occurs through the reduction of NAD+ to NADH H+ –the waste product is CO2. –the product is Acetyl CoA (2C), which can be used in the Kreb's cycle |
|
|
|
What is the product of the link reaction?
|
–2 CO2 per glucose molecule
–2 NADH per glucose molecule –2 Acetyl CoA per glucose molecule |
|
|
|
How can fatty acids be used in cell respiration?
|
–glycolysis is not needed
–fatty acid jumps to the link reaction, where CoA oxidises the long carbon chain of fatty acids. –the reaction is slower |
|
|
|
In the Kreb's cycle, how is citric acid produced?
|
Acetyle CoA (2C) and Oxaloacetate (4C) bind, producing CoA and Citric Acid. The CoA can no bind with another pyruvate in the link reaction.
|
|
|
|
In the Kreb's cycle, how is citric acid turned into a 5 carbon molecule?
|
–Citric acid is oxidized into a 5 carbon molecule, producing a CO2 molecule (this reaction is called decarboxylation) and an NADH.
–A reduction reaction is what causes the oxidation of citrate. NAD+ is reduced to NADH. |
|
|
|
In the Kreb's cycle, how is the 5 carbon molecule turned into a 4 carbon molecule?
|
–The molecule is oxidized into a 4 carbon molecule through:
–The substrate–level phosphorylation of ADP + Pi to ATP –The decarboxylization of CO2 –and the reduction of NAD+ to NADH |
|
|
|
In the Kreb's cycle, how is the 4 carbon molecule modified to create oxaloacetate?
|
–The 4 carbon molecule is modified into oxaloacetate through:
–the reduction of FAD to FADH2 –and the reduction of NAD+ to NADH |
|
|
|
Why is the Kreb's cycle a cycle?
|
It starts and ends with the same molecule: oxaloacetate, so the process can keep repeating over and over.
|
|
|
|
How many CO2, NADH, ATP, and FADH2 are produced from the Kreb's cycle per glucose molecule?
|
–4 CO2 (2 per spin)
–6 NADH (3 per spin) –2 ATP (1 per spin) –2 FADH2 (2 per spin) |
|
|
|
Why is it important that NADH and FADH2 are produced from the Kreb's cycle?
|
They are like the tickets to make ATP in the ETC. They carry H+ ions and electrons which are responsible for the ETC in the cristae.
|
|
|
|
What is the first step of oxidative phosphorylation?
|
Electron transport chain:
–NADH is oxidized, releasing 2 electrons into the inner membrane and a protein pumps 2 hydrogen ions into the intermembrane space –the electron jumps between integral proteins like ubiquione and cytochrome III, following the energy gradient. –Energy is released from the electrons as they move down –This activates protein channels which pump H+ ions through the cristae into the intermembrane space. |
|
|
|
When does FADH2 come into play in electron transport chain?
|
–FADH2 is used only at lower energy levels along ETC and donates 2 electrons as well.
|
|
|
|
What is the second step of oxidative phosphorylation?
|
Chemiosmosis:
–Pumping of carrier proteins (activated by electons moving along the electron transport chain) cause a high H+ conc. in the intermembrane space –Osmosis: areas of high conc. want to move to areas of low conc. –H+ ions flow through ATP synthase enzyme, causing it to spin. –Spinning ATP synthase catalyzes the phosphorylation of ADP + Pi to make a maximum of 34 ATP |
|
|
|
How can chemiosmosis be compared to a dam?
|
–The inner membrane is the damn
–The ATP synthase enzyme is the turbine –The H+ in the intermembrane space is the high water level on one side of the dam –The H+ in the matrix is the low level water level on the other side of the dam –When water spins the turbines, energy is created –When H+ spins the ATP synthase, ATP is created |
|
|
|
On average, how much ATP do NADH and FADH2 yeild per glucose molecule in oxidative phosphorylation?
|
1 NADH yields 3 ATP. 10 NADH produce 30 ATP
2 FADH2 yeilds 2 ATP. 2 FADH produce 4 ATP This amounts to 34 ATP produced by oxidative phosphorylation. |
|
|
|
Why is oxygen important in the ETC?
|
Oxygen is the terminal electron acceptor. It maintains the concentration gradient of H+ and removes electrons by forming water from 1/2 O2 + 2H+ 2 e–. This needs to happen in order for the ETC to continue.
|
|
|
|
Compare aerobic and anaerobic respiration.
|
Aerobic vs Anaerobic
–greater ATP yeild vs low ATP yield –goes through glycolysis to OP vs goes through glycolysis only –end product is 38 ATP vs end product is lactic acid or ethanol and 2 ATP –glycolysis yields 2 ATP, 2 NADH, and 2 pyruvate vs glycolysis yields 2 ATP, 2 ethanol/lactate, and 2 CO2 –uses oxygen vs doesn't use oxygen –both modes of generating energy that need glucose |
|
|
|
What is the difference between substrate–level phosphorylation and oxidative phosphorylation? Where can both of them be found?
|
???
|
|
|
|
What is the difference between substrate–level phosphorylation and oxidative phosphorylation?
|
SLP vs OP
Occurs in glycolysis and krebs vs occurs due to chemiosmosis Phosphorylation of ATP due to the direct phosphorylation of ADP by an intermediate vs phosphorylation of ADP with Pi and chemiosmosis |
|
|
|
Competitive and non-competitive inhibitors differ in... |
-chemical appearance -where they bind -effect on substrate binding -effect on enzyme activity -effect of increasing substrate on inhibition |
|
|
|
How do competitive and non-competitive inhibitors differ in chemical appearance? |
competitive--chemically similar to the substrate non-competitive--not chemically similar to the substrate |
|
|
|
How do competitive and non-competitive inhibitors differ in where they bind? |
competitive--bind in the active site (binding is reversible) non-competitive--binds in allosteric site (binding is reversible) |
|
|
|
How do competitive and non-competitive inhibitors differ in their effect on substrate binding? |
competitive--block the active site so substrate cannot bind non-competitive--change active site so substrate cannot bind (if it still can, enzyme activity slows0 |
|
|
|
How do competitive and non-competitive inhibitors differ in their effect on enzyme activity? |
competitive--slows enzyme activity non-competitive--slows/stops enzyme activity |
|
|
|
how do competitive and non-competitive inhibitors differ in the effect of increasing substrate concentration? |
competitive--max enzyme activity can be reached with enough substrate. B/c of competition, if substrate out competes inhibitor substrate will win. non-competitive--max enzyme activity can not be reached. b/c no competition |
|
|
|
explain end product inhibition |
end product inhibits the first enzyme to inhibit a metabolic pathway when there is an excess of product. |
|
|
|
What is a real life way in which inhibitors can be used? |
Malaria is caused by plasmodium falciparu an inhibitor can be used on the enzymes of this microbe, killing it. |
|

