![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
383 Cards in this Set
- Front
- Back
|
Where is cholesterol synthesised?
|
In the liver, gonads and adrenal cortex
|
|
|
Is the synthesis of cholesterol hormone regulated?
|
Yes.
|
|
|
What is the chemical equation for the synthesis of cholesterol
|
Acetyl CoA >>HGM CoA reductase>>> cholesterol
This is a multi step process |
|
|
How is cholesterol present in different compartments of cells?
|
In the cell, cholesterol exists as it
In the extracellular compartments cholesterol exists as LDL (low density lipoprotein) |
|
|
What is a lipoprotein?
|
A monolayer of phospholipids which are in a circular layer formation.
|
|
|
What is the function of a lipoprotein?
|
To transport lipids around the body
|
|
|
What are apolipoproteins?
|
Small proteins which sit i the external coat of lipoproteins, giving each type of lipoprotein it's specific properties and structurally stabilise them
|
|
|
What are the purposes of apolipoproteins?
|
-act as cofactors for enzymes which act on lipids inside the lipoprotein
-Ligands for receptors -control the metabolic fate of lipoproteins |
|
|
What are chylomicrons? What do they do?
|
A lipoprotein which is synthesised and broken down in the liver.
It is used to transport triglycerides in the blood stream after a meal (form intestine to liver) |
|
|
Which type of lipoprotein has the highest triglyceride content?
|
Chylomicrons with 88%
|
|
|
What is the function of VLDL?
|
Very Low Density Lipoproteins transport triglycerides from the liver to the tissues between means
|
|
|
What is LDL and what is its funciton?
|
Low Density Lipoprotin
A long life lipoprotein which carries cholesterol to the tissues/liver. -Carries BAD cholesterol |
|
|
What is HDL and what is it's function?
|
High Density Lipoprotein
-It is good -High protein content and low fat content -Transports cholesterol away from tissues |
|
|
What is the difference between a peripheral and an integral protein?
|
Peripheral proteins do not penetrate the bilayer, whereas integral proteins do. Some span the total length of the bilayer.
|
|
|
Is the plasma membrane symmetrical?
|
No, because different molecules are needed to interact with extracelluar components then the molecules needed to interact with intracellular components
|
|
|
Does the plasma membrane move laterally or vertically?
|
They constantly move laterally. Vertical movement is rare
|
|
|
What is the function of the cell membrane?
|
Organisation of complex reaction sequences
Segregation of difference processes and components Important in cell-cell communication |
|
|
Why are membrane proteins important?
|
Adhesion
Transport Signalling Energy transducer |
|
|
What are 'rafts' which are found on the cell surface?
|
They are regions on the cell surface which have different molecular compositions
|
|
|
What is the structure of the plasma membrane?
|

|
|
|
What is passive transport?
|
Diffusion through the membrane along a concentration gradient.
-This continues until an equilibrium is reached |
|
|
What is facilitated diffusion?
|
Diffusion through a transport channel along a concentration gradient
|
|
|
What is active transport?
|
The transport of molecules (via channels or endo/exocytosis) against a concentration gradient.
This requires energy |
|
|
What are aquaporins?
|
Channels which help water move across the membrane when flow rates are very fast
|
|
|
What are Gated Ion Channels?
|
Ion channels which allow the diffusion of certain molecules across a membrane only if a signalling molecule binds to the channel first.
This is for regulation |
|
|
What are the 3 different types of transport channels in facilitated diffusion?
|
Uniporter-transports in one direction
Symporter- Transports 2 different molecules in the same direction. Both molecules must be present Antiporter- Transports 2 different molecules in different directions |
|
|
What are GLUT transporters?
|
Different types of transport channels which facilitate the diffusion of glucose across a membrane
|
|
|
How do GLUT transporters work?
|
1) Carrier protein has a glucose-binding site
2) Glucose binds, causing the protein to change shape 3) Protein thus releases glucose 4) Carrier protein returns to original shape |
|
|
How does glucose diffuse into RBCs?
|
Through a GLUT1 transporter
|
|
|
What is the difference between primary and secondary active transport?
|
Primary active transport involves the direct hydrolysis of ATP
Secondary active transport gets its energy from the chemical and electrical gradients already establisedh by primary active transport |
|
|
How does the Sodium-Potassium pump work?
|
1) ATP and Na+ bind to pump
2) ATP hydrolysed, this phosphorylates the protein 3)Shape change causes Na+ to move outside the cell and K+ binds to the pump 4)Pi is released and transporter molecule returns to its original shape 5)K+ is released into the cytoplasm |
|
|
When is glucose transported across membranes via secondary transport?
|
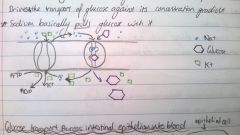
It occurs in the gastrointestinal tract, when Glucose moves into the epithelial cells from the lumen
-Na+/K+ pump establishes a concentration gradient -Na+ moves back across the concentration gradient, pulling glucose with it (Na+/Glucose symporter) |
|
|
How is glucose transported into the blood stream from the lumen of the GIT?
|
1) Na+/Glucose symporter into epithelial cell (driven by hight Na+ in the lumen)
2)GLUT2 uniporter moves glucose into the blood stream (passive) 3) Na+ is moved back out by the Na+/K+ antiporter (ATP) |
|
|
What is the equation for the transport of carbon dioxide from tissues to expulsion? What chemicals are present where?
|
CO2 + H2O <> HCO3- + H+
CO2-in respiring tissue HCO3- in blood CO2 -leaving lungs |
|
|
What is pinocytosis
|
The process of taking liquids into a cell via endocytosis
|
|
|
What is Receptor mediated endocytosis?
|
Endocytosis where a particular ligand/vesicle has to bind to a receptor on the cell before transport occurs
|
|
|
Explain glycogenesis
|
A process where glycogen is synthesised in the liver.
-Pathway activated after an energy dense meal -Requires the presence of glycogenin (primer enzyme) -Glucose enters hepatocytes via GLUT 2 transporters -Converted to Glucose-6-Phosphate by glucokinase -Converted to G-1-P then UDP glucose -UDP cleaved off, and then added to the growing chain by glycogen synthase |
|
|
Explain liver glycogenolysis
|
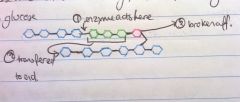
A process that maintains blood glucose levels
-Glycogen phosphorylase breaks a1-4 glycosidic bonds -Cannot break them 4 molecules away from branch point -Glucosidase cleaves a1-6 bond -Transferase attaches the branch onto the end of the unbranched chain -Process continues, mobilising glycogen for energy use |
|
|
When is insulin released and what does it do?
|
Released when blood sugar levels are high
-Stimulates glycogen synthase -Inhibits glycogen phosphorylase |
|
|
When are glucagon and epinephrine released and what do they do?
|
Released when blood-glucose is low
-Stimulates glycogen phosphorylase -Inhibits glycogen synthase |
|
|
What are the 2 enzymes which regulate the addition and removal of phosphate groups?
|
Kinase-adds
Phosphotase-Removes |
|
|
Glycogen synthase (producer of glycogen) can be phosphorylased and unphosphorylased. When is it most active?
|
When it is not phosphorylased
|
|
|
Glycogen Phosphorylase (breaks down glycogen) can be phosphorylased or unphosphorylased. When is it most active?
|
When it is phosphorylased
|
|
|
Explain gluconeogenesis
|
The synthesis of glucose after long periods of fasting when blood glucose levels are low.
-Essentially reverse-glycolysis, except for 3 steps which cannot be reversed -Pyruvate is ultimately converted back into glucose |
|
|
What are some features of gluconeogenesis?
|
-Slower response than the breakdown of glycogen
-Primary source of blood glucose 8 hours after absorption -requires carbon skeletons |
|
|
Why doesn't the conversion of lactate back into glucose result in a net gain of glucose?
|
Because lactate originated from glucose before anaerobic respiration occured
|
|
|
How are amino acids used in GNG?
|
Transaminase use used to remove the amino group from the AA
The remaining carbon skeleton can be turned into glutamate or alanine via transamination to be used in GNG after being turned into intermediates of cellular respiration |
|
|
What is the difference between alanine and glutamate when they are used in gluconeogenesis?
|
Alanine is transported to the liver. Converted to pyruvate then into glucose
Glutamate is converted to glutamine and transported to the kidneys and is converted into glucose |
|
|
What are the 2 major processes of amino acid catabolism?
|
-Removal and excretion of the amino group. (trans and deamination)
-Oxidation of the carbon skeleton |
|
|
Explain transamination
|
Transaminases and aminotransferases catalyse the transfer of amino groups
-a ketoglutarate acts as an acceptor - L-glutamate is formed -Requires pyridoxal phosphate |
|
|
Explain Deamination
|
Different enzymes are required for different AAs
-Ammonia is released from the AA into the liver -Ammonia enters urea cycle (Ony in the liver) -Ammonia converted into urea for ixtretion |
|
|
Explain the urea cycle
|
-An energy requiring process (3ATP)
-2NH4+ and HCO3- are converted into Urea |
|
|
What are glucogenic amino acids?
|
Amino acids which are converted into glucose in the liver
|
|
|
What are keytonic amino acids?
|
Amino acids converted to keytone bodies by the liver
|
|
|
How does the liver metabolise drugs/toxins?
|
1) Compount is modified to become hydrophilic for easier excretion
-this can make it more reactive 2)Conjugation renders the compound more soluble and less toxic |
|
|
What are xenobiotics?
|
Compounds which are foreign to an organism
-drugs, carcinogens, pesticides etc |
|
|
What is the primary energy source 0,30 and 60 minutes into a race?
|
0- glycogen, but often anaerobic respiration occurs
30-fats and carbs 60- fats |
|
|
How does alcohol affect the glycolytic pathway?
|
-Decreased rate because of a build up of Acetyl CoA and decreased [NAD+]
|
|
|
Why might someone be hypoglycaemic and have lactic acidosis after excessive alcohol consumption?
|
-Pyruvate is converted into lactic acid, which can build up and end up in the blood stream
-Decreased levels of pyruvate lead to a low rate of gluconeogenesis and therefore a low blood-glucose level |
|
|
Why might someone be treated with bicarbonate after consuming too much alcohol?
|
-A large amount of H+ ions are produced in the conversion of NAD+ to NADH
-HCO3- reacts with H+ to bring blood pH back to normal |
|
|
Why might thyamine be added to a drip after excessive alcohol consumption/alcohol abuse
|
-Person could be thyamine defficient, a product which is essential cofactor for alcohol dehydrogenase
-Poor diet as alcohol is the primary energy source -Alcohol decreases absorption of essential nutrients |
|
|
How would alcohol consumption affect fatty acid metabolism?
|
It would create a build up of fatty acids because a lack of NAD+ drives the synthesis of fatty acids forward.
Acetyl coA builds up and is converted into fatty acds for the same reason. |
|
|
Why does excess alcohol consumption lead to hyperlipidaemia?
|
High concentrations of fatty acids in the liver lead to a secretion of large quantities of Fatty Acids in the blood stream
|
|
|
Explain the biochemical basis of why someone is tired after lots of alcohol?
|
Reduced level of ATP production because of the decreased NAD+ levels leading to a slow down of the CAC
Also, hypoglycaemia and poor nutrition can be factors |
|
|
Discuss the microsomal ethanol oxidising system (MEOS)
|
It is an alternate pathway of alcohol metabolism
Uses cytochrome p450, which has a high affinity for alcoho Occurs in the endoplasmic reticulum of liver cells Occurs in conjunction with regular alcohol metabolism to speed up the process Induced by high levels of ethanol Can create harmful free-radicals |
|
|
What are adipocytes?
|
Cells which store triglycerides and make up adipose tissue.
|
|
|
How much oxygen does skeletal muscle produce at rest and during activity?
|
At rest: 20%
During activity 90% |
|
|
What sources of energy does skeletal muscle use at rest?
|
Fats and keytone bodies
|
|
|
What sources of energy does skeletal muscle use when it's moderately active?
|
Glucose from glycogen stores
Fatty acids Keytones |
|
|
What source of energy is used by skeletal muscle during short bursts of energy
|
ATP reserves
Creatine phosphate- |
|
|
What is creatine phosphate?
|
A high energy storage compound which regenerates ATP during the first few minute of energy requirements
|
|
|
What energy sources are used by skeletal muscle during levels of extreme activity?
|
Mostly ATP, but there isn't enough oxygen for this to occur aerobically.
|
|
|
What energy sources are used by skeletal muscle during starvation?
|
Proteins are broken down, transported to the liver, then used to produce energy there
|
|
|
Can heart muscle undergo anaerobic respiration?
|
No
|
|
|
How does heart muscle store energy sources
|
It doesnt. It takes up glucose, keytones and lipids directly from the blood stream
|
|
|
What source of energy does the brain use?
|
Blood glucose and keytones
|
|
|
Does brain tissue have fuel reserves
|
No
|
|
|
How do RBCs get an energy supply?
|
They have no organelles or mitochondria
Glucose is the #1 source of energy Metabolism is entirely anaerobic so that all the O2 is passed on to other cells |
|
|
What is the transforming principle/factor of organisms?
|
DNA
|
|
|
What are the components of nucleic acids?
|
Pentose Sugar
Nitrogenous bases Phosphate |
|
|
What is Chagraffs rule?
|
There are equal amounts of A and T and equal amount of G and C
Number of purines=number of pyramidines |
|
|
Which way does a DNA double helix twist?
|
Right
|
|
|
What does antiparallel mean and how is it relevant to DNA?
|
aniparallel means that the strands run in different directions. This is due to the complementary base-pairing system
|
|
|
How many bonds are there between AT and GC
|
AT=2 H bonds
GC= 3 H bonds |
|
|
Which carbon does the phosphate link to in DNA?
|
The 3' on the first and the 5' on the second
|
|
|
What are chromosomes made of?
|
Protein and DNA
|
|
|
What are nucleosomes?
|
146 base pairs wrapped around a core of 8 histone molecules. 2 of each 4 types
|
|
|
What is chromatin?
|
Folded nucleosome complexes
|
|
|
Why do some RNA's appear to be double stranded?
|
Because of complementary base pairing within the RNA molecule
ie) tRNA |
|
|
What is the central dogma of molecular biology?
|
DNA --> RNA --> Polypeptide
|
|
|
What are the exceptions to the central dogma of molecular biology and DNA?
|
Reteroviruses: RNA>DNA>RNA>polypeptide
Some other viruses can just skip the DNA part completely |
|
|
What are introns and exons?
|
Introns are the non-coding regions of DNA
Exons are the coding sequences od FNA |
|
|
What are the promoter and terminator sequences?
|
They are flanking regions which help control transcription
|
|
|
What are pseudogenes?
|
Sequences in gene families that appear almost identical to the functional genes, but a rearely transcribed
ie) the y2 version of the globin gene in foetal development |
|
|
How is RNA synthesised?
|
RNA polymerase II binds to the promotor sequence on DNA
RNA Ploymerase II unwinds the DNA Free nucleotides are used to synthesise RNA in a 5'-3' direction Downstream sequences signal the end of transcription RNA peels away from the DNA, which rewinds pre-mRNA is produced |
|
|
is RNA proof read?
|
No
|
|
|
What does it mean when it is said that the genetic code is redundant?
|
There is more than 1 codon for an amino acid
|
|
|
What does it mean when it is said that the genetic code is non-ambiguous?
|
Each codon is assigned to 1 amino acid
|
|
|
Why is the genetic code considered to be universal?
|
It applies to almost all organisms
|
|
|
How is pre-mRNA processed?
|

-Introns are removed by spliceosomes
-small nuclear riboproteins (snRNPs) bind to the ends of the intron sequence -The introns fall away and bind the exons together -A methyl-G-cap is added to the 5' end A poly-A-tail is added to the 3' end |
|
|
What is the purpose of the methyl-G-cap and the poly-A-tail?
|
They make sure that the ends don't fall apart: They give stability to the structure
|
|
|
What is alternate splicing?
|
Where different combinations of exons are left in the final mRNA to give rise to slightly different proteins
|
|
|
Where does the energy driving the transcriptional process come from?
|
The cleavage of 2 phosphate groups off ribonucleotide triphosphates
|
|
|
How is gene expression controlled at a transcriptional level?
|
-Transcription factor proteins bind to promoter regions of DNA which is going to be transcribed
-Activation/surpressor proteins can alter the initiation of transcription |
|
|
How is gene expression controlled at a post-transcriptional level?
|
-Alternative splicing
-AU Rich sequences promote rapid breakdown of the mRNA -miRNA binds to the mRNA , preventing translation and facilitating degradation |
|
|
How is gene expression regulated at a translational level?
|
Activator and repressor proteins bind to mRNA, determining which and how much protein is translated
|
|
|
How is gene expression regulated at a post-translational level?
|
-Regulation of protein lifespan. Ubiquitin binds to the protein, marking it for destruction. The protein binds to proteasome which breaks it down
|
|
|
What is the difference of gene expression between in prokaryotes and eukaryotes?
|
Gene expression is quicker in prokaryotes because all processes take place in the cytoplasm
|
|
|
What is the wobble theory?
|
When the tRNA binds to mRNA at the ribosome, there is allowed to be some ambiguity in the 3rd base of the anticodon.
There are less tRNA molecules than codons. This interaction does not take place all the time |
|
|
What are the key features of tRNA?
|
-Anticodon which is complementary to DNA for interactions
-Amino acid attachment site- a hydroxyl group -Single stranded RNA which folds in on itself |
|
|
How do amino acids bind to their tRNA molecules?
|
The enzyme Aminoactyl tRNA synthetase forms an ester bond between the AA and the tRNA
|
|
|
What is a 'charged' tRNA molecule?
|
One that has it's AA bonded to it
|
|
|
What is the structure of a ribosome?
|
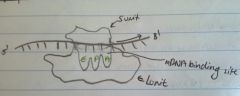
2 subunits
L subunit (large) has 3 RNA sites S subunit (small) has 1 RNA site |
|
|
Are the ribosome subunits together all the time?
|
No, they are only together when translating
|
|
|
What bonds hold the subunits of ribosomes together?
|
Ionic, and hydrophobic bonds
|
|
|
What are the binding sites (in order) inside the ribosome?
|
A(amino acid)
P(polypeptide) E(Exit) |
|
|
What does the A site do in the ribosome?
|
The tRNA anticodon binds to the mRNA codon
|
|
|
What does the P-site do in the ribosome?
|
The site where tRNA adds its amino acid to the chain
|
|
|
What does the E site do in the ribosome?
|
It's where the uncharged tRNA molecule leaves the ribosome
|
|
|
What are the 3 stages of translation?
|
Initiation
Elongation Termination |
|
|
Describe the initiation phase of tranlation
|
The initiation complex forms
-First charged tRNA (methionine), mRNA and S subunit form -L subunit joins on -Process is driven by initiation factors (Use GTP) |
|
|
Describe the process of elongation
|
-Peptidyl transferase breaks the bond between AA and tRNA
-This gives the energy for the polypeptide chain to move from the P trna to the Atrna -The uncharged tRNA leaves the ribosome and the process continues -Chaperones ensure correct folding |
|
|
Describe the process of termination
|
A stop codon reaches the A site
A release factor enters instead of an AA Translation terminates and the polypeptide falls away |
|
|
What are the 3 main methods of proofreading and repair of DNA?
|
Proofreading mechanisms
Mismatch repair mechanisms Excision repair mechanisms |
|
|
What happens in the proofreading mechanism of DNA repair?
|
DNA polymerase II corrects errors as replication is occuring
|
|
|
How does DNA repair come about in mismatch repair mechanisms
|
DNA polymerase I scans and repairs errors shortly after transcription
|
|
|
How does DNA repair work in excision repair mechanisms?
|
DNA polymerase I repairs errors over the full lifetime of the cell
-Repairs errors sustained from chemicasl or radiation |
|
|
What is cisplatin?
|
A drug that forms covalet cross links between DNA strands, inhibiting DNA replication
|
|
|
Describe DNA replication
|
-Begins a the origin of replication where DNA helicase denatures and unwinds the double strand
-RNA polymerase catalyses a short primer region -DNA polymerase III catalyses the addition of nucleotides in a 5'-3' sequence -Energy from the conversion of a dNTP to a nucleotide is used -RNA primer is degraded and replaced with DNA |
|
|
How are DNA strands kept apart during replication?
|
proteins bind to DNA strands
|
|
|
What is the difference in the leading and lagging strands in DNA replication?
|
The leading strand synthesises continuously in a 5'-3' direction
The lagging strand synthesises in 5'3' direction, but does so in fragments because of the different orientation of the strand. |
|
|
Explain the replication process on a lagging strand
|
short, okazaki fragments are added onto the primer ends and DNA polymerase III skips past the 5' end and goes on to synthesise the next fragment
|
|
|
What is the role of Ligase and DNA polymerase I in DNA replication
|
DNA polymerase I replaces the RNA primer with DNA
Ligase seals the gaps between okazaki fragments |
|
|
What happens at the end of DNA replication in chromosomes?
|
There is no place for primers to bind at the end of a c'some so after each replication, little sections of DNA are cut off
|
|
|
What are telomeres
|
They are repetitive sequences of DNA at the end of chromosomes which protect the functional DNA from degradation.
|
|
|
What happens when Telomeres are too short?
|
The telomeres are no longer longe enough to protect the DNA, the cell is deemed too old and is signalled to undergo apoptosis
|
|
|
What is the function of telomerase?
|
It rebuilds the telomeres of constantly dividing cells
|
|
|
What are ddNTPs?
|
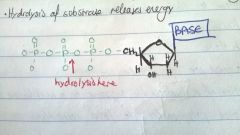
dideoxynucleoside triphosphates
Lack an OH group on the 3' end |
|
|
How can ddNTPs be used in nucleotide sequencing?
|
-Many copies of a single strand of DNA are mixed with DNA polymerase, primers and dNTPs and small amounts of ddNTPs, which are fluorescently tagged.
-The ddNTPs do not have a hydroxyl (OH) group at the 3' end and this means that DNA synthesis will stop when a ddNTP is incorporated because no nucleotide can be added on. -DNA polymerase synthesises the new strand of DNA mostly from the normal nucleotides but a ddNTP is sometimes randomly used and thus, different lengths of double stranded DNA are produced -Gel electrophoresis is used to separate out these different lengths and a laser is used to read the fluorescently tagged nucleotide at the end of each sequence in order to determine the sequence of the original single strand of DNA |
|
|
What are the only cells capable of undergoing mitosis and meiosis?
|
Germ Cells
|
|
|
What is a primordial germ cell?
|
A cell which is protected from differentiation so that it can later become a gamete cell
|
|
|
When does the major portion of germ cell occur?
|
during embryonic development
|
|
|
What are the 4 stages of the primordial cell journey?
|
Specification- When cells express a gene that makes them considered to be a germcell line
Commitment Migration- migration to gonad location, extensive proliferation Colonisation-PGCs colonise gonads |
|
|
What is passive migration in germ cell development?
|
The migration of germ cells from the embryonic midline to the gonads which is permitted by the shape change and development of the embryo.
|
|
|
What is active migration during germ cell development?
|
Where the proliferation of PGCs occurs during migration
|
|
|
During gamete development, when does the sex-determination stage occur?
|
During week 6
|
|
|
What happens in week 6, sex determination of a cell line, that can seriously disrupt development?
|
Genetic malformations
When development doesn't go right, the gonocytes of males don't form properly, and can later lead to prostate cancer |
|
|
What is the SRY gene?
|
A sex-determining gene which is located on the Y c'some
|
|
|
How does the SRY gene control sex determination?
|
The SRY gene is present in males, and represses the hormone signalling for the development of the female duct system.
|
|
|
What is the difference between male and female meiosis?
|
-Spermatogensis produces 4 mature, equal spermatids whereas Oogenesis produces only 1 egg and 3 polar bodies
-The times of germ cell maturation differ |
|
|
What are the similarities of male and female meiosis?
|
They both have an incapacity to survive for long if fertilisation does not occur
They both undergo significant morphological differentiation |
|
|
Describe the process of male germ cell development
|
Male germ cells form gonocytes which mature into intermediate spermatogonia at birth.
After puberty, spermatogenesis occurs rapidly and constantly, giving rise to 4 spermatids |
|
|
Describe the process of female germ cell development
|
Germ cells differentiate into oogonia which arrest in prophase I of meiosis @ week 12. Oogenesis is asymmetrical, only giving rise to 1 egg.
At puberty FHS stimulates follicle growth. Meiosis resumes a few hours before ovulation |
|
|
Which cells regulate the hormonal control of oogenesis?
|
Granulosa cells-
Theca cells- produce lutenising hormone |
|
|
Which cells regulate the hormonal control of spermatogenesis?
|
Leydig cells- Testosterone
Sertoli Cells- Express SRY and form the blood/testis barrier |
|
|
Where in the testes does spermatogenesis occur?
|
In the seminiferous tubes. The sperm are released into the lumen of that tube and emptied into the vas deferens
|
|
|
What is the syncytium?
|
A link between the spermatids which allows them to stay connected with one another to stay in supply of X-c'some products.
|
|
|
What happens during follicle growth that leads to only 1 egg out of 50 finishing maturation?
|
One follicle becomes dominant and becomes Follicle Stimulating Hormone independent. It secretes inhibin which degenerates the remaining follicles.
|
|
|
Describe meiosis 1
|
Prophase: Chromosomes condense, synapsis occurs, crossing over occurs
Metaphase: Homologous pairs line up along the equatorial plate. Independent assortment occurs Anaphase :Homologous chromosomes are pulled apart by the shortening of the spindle fibres Telophase: 2 haploid sets are formed SISTER CHROMATIDS REMAIN TOGETHER, THIS IS WHY THE CELL IS HAPLOID, NOT DIPLOID |
|
|
What is synapsis?
|
The fusion of homologous pairs to form bivalents
|
|
|
Describe Meiosis 2
|
Prohase: Chromosomes condense, and centrioles move to cell poles. Cells migrate towards equator
Metaphase: Cells line up along the equatorial plate Anaphase: Sister chromatids separate Telophase: 4 genetically dissimilar, haploid gametes are formed |
|
|
What is the order in which you classify epithelial tissues?
|
1) Number of Cell layers
2) Cell Shape 3) Surface specialisations |
|
|
What are the classifications of cell layers in epithelial cells?
|
Simple-single layer
Stratified - multiple layers Pseudostratified - Appearance of being multiple layers but all cells are connected to the basal layer |
|
|
What are the classifications of cell shape in epithelial cells?
|
Squamous- flat
Cuboidal- Square/rounded Columnar- long |
|
|
What are the different types of surface specialisations which can occur in epithelial cells?
|
Keratin
Cilliated/microvilli |
|
|
What is transitional epithelium?
|
Epithelial cells which can slide over the top of one another and reaggregate in order to stretch and relax
|
|
|
What is the difference between and endocrine and an exocrine gland?
|
Endocrine glands secrete substances into ducts (Sebaceous gland)
Exocrine Glands secrete substances into blood/tissue etc |
|
|
What is the only junction not present in epithelial cells?
|
Gap junctions
|
|
|
What is connective tissue made up of?
|
Cells
Fibre Extracellular matrix |
|
|
What are the 3 major cell types in connective tissues?
|
Fibroblasts- make collagen
Chondrocytes- make cartilage Osteoblasts- make bone Adipocytes-store lipids |
|
|
What are the 5 different types of connective tissue?
|
Loose CT
Dense CT Cartilage Bone Blood |
|
|
What is loose connective tissue?
|
Has a large portion of cells and cell matrix rather than fibres which is loosely arranged
-Good for packing and protecting |
|
|
What are 3 major types of loose connective tissue
|
Areolar CT: Attaches skin to underlying components, surrounds blood vessels and organs
Adipose CT: Fat storage Reticular CT: Supports liver, lymphoid and bone marrow tissue |
|
|
What are the 2 types of dense connective tissue?
|
Dense regular and dense irregular
|
|
|
What is the function of Dense Regular CT?
|
It has the ability to oppose great force from one direction because of it's fibres being in a parallel arrangement
|
|
|
What is the function of dense irregular CT?
|
It can oppose force from many directions because it's fibres are arranged in such a way as to do this
|
|
|
Why does elastic cartilage appear blackish on a H&E stained slide?
|
Because of it's hight content of dense elastin fibres
|
|
|
What are the major components of blood?
|
RBC, leukocytes, platelets and plasma (the liquid matrix)
|
|
|
What are the 3 types of muscle tissue?
|
Skeletal, smooth and cardiac
|
|
|
What are the features of skeletal tissue?
|
-Parallel muscle fibres
-Multinucleated -Striations -Voluntary movement |
|
|
What are sarcomeres?
|
The contractile unit of cardiac and skeletal muscles
|
|
|
Is skeletal muscle able to be regenerated?
|
Yes, to an extent
|
|
|
What are the key features of cardiac muscle?
|
-Single, central nucleus
-Branched -Joined by gap junctions -Involuntary movement -Striated |
|
|
Is cardiac muscle able to be regenerated?
|
no
|
|
|
What are the key features of smooth muscle?
|
-Spindle shaped
-Unstriated -Closely packed |
|
|
Where is smooth muscle often found?
|
Lining organs where shape changes are required. Often lining hollow organs
|
|
|
What are the features of smooth muscle contractions?
|
They are slow and involuntary
|
|
|
What is an efferent neuron?
|
A neuron that sends messages from the CNS to the muscle
(Motor) |
|
|
What is an afferent neuron?
|
A neuron that sends messages from the muscles to the CNS
(Sensory) |
|
|
What are dentrites?
|
Projections which carry action potentials to the cell body
|
|
|
What is another name for the cell body?
|
The soma
|
|
|
What is an axon?
|
A projection from the cell body that carries the electric impulse/message to other cells
|
|
|
What is the function of myelin?
|
It is an insulator of electric impulse that makes impulse conduction faster
|
|
|
What are synapses?
|
Gaps which allow communication and connection between nerve cells
|
|
|
What are glial cells?
|
Aka neuroglia
Supporting cells which do not participate directly in impulse transmission |
|
|
What are the 3 main types of neuroglial cells in the CNS?
|
Astrocytes
Oligodendrocytes Microglia |
|
|
What is the difference between white and grey matter in terms of their cell content?
|
White matter has axons and dendrites
Appears white because of the myelin Neuron cell bodies are only found in grey matter |
|
|
What are astrocytes?
|
Starshaped cells which form part of the blood-brain barrier.
|
|
|
What is the difference between fibrous and protoplasmic astrocytes?
|
Fibrous astrocytes are found in grey matter and protoplasmic astrocytes are found in white matter
|
|
|
What is the function of an oligodendrocyte?
|
They create myelin in the CNS
|
|
|
What is the function of Microglia?
|
They make up part of the immune defense system in the CNS
They are the macrophages of the CNS |
|
|
What is the difference between schwann cells and oligodendrocytes?
|
Schwann Cells give rise to myelin in the PNS
Oligodendrocyttes give rise to myelin in the CNS |
|
|
What is nephrogenesis?
|
The development of the nephrons and kidneys
|
|
|
What is a major feature of nephrogenesis in terms of the interaction of different tissue growths. What is the name of this feature?
|
One part of the growing organ induces another part to turn into a specialised component: The filtration and excretion parts.
The Reciprocal Induction of Development |
|
|
How does the reciprocal induction of development come about in the kidney?
|
A small primitive bud develops from the mesoderm in the pelvis area
The bud secretes growth factors which stimulate branching morphogenesis These cells will form the ducting and excretion system of the kidney |
|
|
What are the 2 major phases and the 4 subphases of the cell cycle?
|
Major: interphase and mitosis
Subphases G1, S, G2, Mitosis, Cytokenesis |
|
|
What is the role of growth factors in the cell cycle?
|
They determine whether or not a cell will proliferate/continue to proliferate. It controls when cells go into G0
|
|
|
What is G0?
|
A phase where the cell is not in the cell cycle and is at 'rest'
|
|
|
What is the purpose of cell cycle checkpoints?
|
They prevent the next phase of the cell cycle from occurring until the events of the previous phase have been completed
|
|
|
which enzymes control the cell cycle?
|
Kinases, which are activated by cyclins
|
|
|
When is cyclin the most active in the cell cycle?
|
When mitosis is occuring: Sufficient levels of cyclin must be present for mitosis to even occur
|
|
|
What is apoptosis?
|
Programmed cell death which is a genetically related death carried out by a series of events
|
|
|
What are the 3 tumour suppressor genes?
|
P53, P63 and P73
|
|
|
What is the purpose of tumour suppressor genes?
|
P53 usually arrests the cell cycle to let DNA repair or induces apoptosis if damage is too severe to repere
|
|
|
What happens when tumour suppressor genes don't work?
|
If the genes mutate/don't work, apoptosis won't occur when it's really needed and cells will go on to accumulate more mutations and eventually form cancer
|
|
|
What is the process of apoptosis?
|
1) signals are recieved
2) Cell shrinks and looses contact with neighbouring cells while chromatin begins to break down 3) Nuclear membrane degrades and 'blebs' form on the surface 4) Nucleus collapses and cell breaks up into apoptotic bodies which are phagocytosed |
|
|
What is necrosis?
|
What a cell is destroyed due to irreparable trauma. The cell will swell and burst and contents leak out
|
|
|
What is a pedigree?
|
A family tree which shows the history of a certain trait
-Begins with the index case (who had it first), indicated with an arrow |
|
|
What are Mendelian disorders?
|
Single gene disorders
|
|
|
What is the difference between a dominant and recessive trait
|
Dominant traits will be expressed preferentially in a heterozygous state while recessive traits will be expressed only in their homozygous state
|
|
|
What is pleiotrophy?
|
When one gene gives rise to 2 or more traits
|
|
|
What is penetrance?
|
The percentage of people who have a gene and express the characteristic of a gene.
Incomplete/non-penetrance is when less than 100% of individuals express a trait |
|
|
What is pseudodominance?
|
When a person with a recessive disorder has children with a person who is a carrier of that disorder.
|
|
|
What is it meant by being hemizygous
|
Having single copies of a gene in an otherwise diploid genome
-Males XY genes |
|
|
What is X inactivation?
|
The random suppression of genes on one X chromosome in somatic cells
|
|
|
Why is X inactivation so important?
|
Without it, there would be an over-expression of genes from the X chromosome.
|
|
|
What is the difference between induced and spontaneous mutations of DNA?
|
Induced mutations occur after exposure to mutagenic agents whereas spontaneous mutations are mutations which occur as a result of errors in DNA replication and repair
|
|
|
What is the difference between somatic and germline mutations?
|
Somatic mutations can be passed onto cell lines via mitosis but germline mutations are passed onto the next generation
|
|
|
What are the different phenotypic effects of mutations?
|
Silent- no effect
Loss of function- function of protein is altered Conditional mutation- A mutant allele causes a mutant phenotype only under certain conditions |
|
|
What are the permissive and restrictive conditions?
|
The restrictive condition is the condition under which a conditional mutation is expressed
The permissive condition is the condition under which the wild type phenotype is expressed |
|
|
What are the different types of point mutations?
|
Silent
Missense Nonsense Frameshift |
|
|
What is a silent mutation?
|
One that has no effect on the protein
-redundancy of the genetic code -occurs in non-coding region of DNA |
|
|
What is a missense mutation?
|
one that alters the structure of a protein
-Reduces efficiency -Can over-function |
|
|
What is a nonsense mutation?
|
One that codes for a STOP codon too early
|
|
|
What is a frameshift mutaion
|
One where all codons for amino acids are changed past the mutation point
-deletions or insertions |
|
|
What are some chromosomal mutations?
|
Deletions
Duplications Inversions Translocations |
|
|
What is a disorder of haemoglobin OTHER than sicke cell anaemia?
|
Thalassemia
|
|
|
What is Thalassemia?
|
When there is an imbalance in the number of alpha/beta globin chains produced due to missing genes
|
|
|
What are the types of a-thalassemia?
|
1 gene missing-carrier
2 genes-mildly affected 3 genes- strongly affected 4 genes- spontaneous abortion |
|
|
What are the types of b-thalassemia?
|
1 gene affected- mildly affected
2 genes - strongly affected |
|
|
What is the P arm of a chromosome?
|
The short arm
p=petite |
|
|
What is the q arm of a chromosome?
|
The long arm
|
|
|
What is a metacentric chromosome?
|
One where the centromere is in the middle
|
|
|
What is a acrocentric chromosome?
|
One where centromeres are at 1 end
-satellites are present |
|
|
What is a submetacentric chromosome?
|
One where the centromere is mostly to the top
|
|
|
What a homologues?
|
members of a pair of chromosomes
|
|
|
What is the order of a karyotype description?
|
-No c'somes
-Sex C'some make up -Description of abdormality 46, XX, del(5p) female,deletion on short arm of c'some 5 |
|
|
What is an ideogram?
|
A stylised version of a karyotype used for standardising and numbering banding patterns. This allows an accurate description of abnormalities
|
|
|
What is aneuploidy?
|
The loss/gain of 1 or more c'some
Monosomy Trisomy Tetrasomy |
|
|
What are 3 examples of aneuploidy syndromes?
|
Down syndrome 21
Patau Syndrome 13 Edwards Syndrome 18 |
|
|
What is polyploidy?
|
Multiple sets of chromosomes in cells
This is normal in megakaryocytes though |
|
|
When might polyploidy occur?
|
Fertilisation of egg by 2 sperm
fertilisation of egg by diploid sperm Fertilisation of diploid egg |
|
|
How can Down syndrome/aneuploidy result?
|
Non disjunction of chromosomes in Meiosis 1 or 2
Robertsonian translocations During the early mitotic divisions after fertilisation |
|
|
What is mosaicism?
|
When aneuploidy occurs in SOME cell lines as a result of non-disjunction in a mitotic division after fertilisation.
|
|
|
What are 3 types of translocations?
|
Robertsonian
Reciprocal Insertional |
|
|
What is a robertsonian translocation?
|
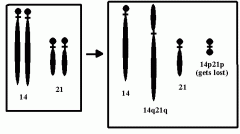
When the short arm of 2 acrocentric c'somes is lost
|
|
|
What is a reciprocal translocation
|
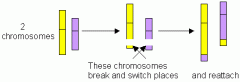
When segment of non-homologous c'somes are exchanged
|
|
|
What is an insertional translocation?
|
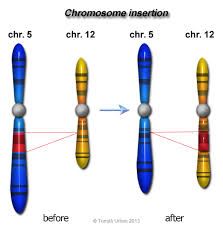
When a segment in the middle of a c'some breaks off and inserts itself into the middle of a non-homologous chromosome
|
|
|
What is polygenic inheritance?
|
When several different genes contribute to the expression of a trait
|
|
|
What is a multifactorial trait?
|
One where multiple genes are influenced by the environment to produce a specific trait
|
|
|
What is the difference between continuous and discontinuous multifactorial traits?
|
Continuous has a normal distribution while discontinous traits mean that a person is either affected or not affected
|
|
|
What are the characteristics of multifactorial diseases?
|
Diseased children can have normal parents
The more sever a disease the higher the recurrence risk less environmental risks present =less overall risk |
|
|
How can the recurrence risk of a multifactorial disease be determined for a population?
|
Approx recurrence risk of pop. = sq.root(population incidence)
|
|
|
What is heritability?
|
An estimate of the proportion of variability of a genetic trait
|
|
|
What is gene epidemiology?
|
Identification of the exact genes which contribute to the etiology of a disease
-Done with the help of twin studies |
|
|
What are dischordant traits
|
Those which are not shared by individuals
|
|
|
What is epistasis?
|
A gene interaction where 1 allele at a locus alter the physical expression of a non-allelic gene
-Can affect 1 or more genes eg) suppressor genes |
|
|
What is allele frequency?
|
Number of copies of an allele / Sum of alleles in population
2NAA + NAa / 2N |
|
|
What is the genotype frequency of a population?
|
No. people with a phenotype / total population
|
|
|
What is the difference between allele frequency and genotype frequency?
|
Allele freq. shows only the frequency of a specific allele while gene frequency shows the proportion of a genotype in a population
|
|
|
What is the Hardy Weinberg principal?
|
Applied to large populations where
-there isnt migration -there are no mutations -no selective breeding/natural selection |
|
|
How can you figure out genotype frequency from allele frequency?
|

Using the rule p^2 + 2pq + q^2=1
where p = A, q=a p^2 is the disease incidence |
|
|
What is gene flow?
|
change in allele frequencies due to migration
|
|
|
What is genetic drift?
|
Change in frequencies due to random sampling
|
|
|
What is consanguinity?
|
Inbreeding
|
|
|
What is Gel Electrophoresis?
|
-Gel electrophoresis is the process by which lengths of DNA are separated based on their sizes. Restriction enzymes are used to cut DNA at specific -base sequences.
-Once the DNA is cut, it is placed into the wells in the gel matrix at the negative end of the electric current. -An electric current is passed through the gel matrix and as DNA is negatively charged, it will be drawn towards the positive end of the current flow. -Different sized DNA fragments will move at different speeds (smaller= faster, larger= slower) which creates the banding patterns which show separation of these fragments |
|
|
Explain PCR
|
1) double stranded DNA is heated to 95 degrees to denature it
2) it's cooled to 60 degrees and primers attach to the two strands 3)Heated to 72 degrees: optimal temp for taq polymerase 4) Taq Polymerase extends the primers making a complementary DNA strand This is repeated until the required about of DNA is met |
|
|
What are DNA microarrays?
|
DNA microarrays are created by robotic machines that arrange minuscule amounts of hundreds or thousands of gene sequences on a single microscope slide.
|
|
|
What is the use of DNA microarray technology?
|
It can determine which genes are switched on or off in a cell.
-RNA molecules are collected -mRNA is isolated and cDNA is synthesised via reverse transcriptase -cDNA is tagged -cDNA is placed onto the microarray slide -cDNA will hybridise with the genes -the number of hybridisations indicates which genes are switched on and which are switched off |
|
|
How can DNA microarrays be used to determine which genes are on/off in cancer and normal cells
|
cDNA from both samples are tagged with different colours and mixed on the microarray slide together.
Different colours indicate which genes are switched on when. |
|
|
How does gene cloning work?
|
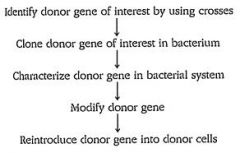
|
|
|
What is a vector?
|
A foreign agent used to insert DNA into a cell
-commonly a retrovirus or a plasmid |
|
|
How is recombinant DNA introduced into a cell?
|
Biological (virus), chemical (treatments which induce membrane to open up, liposomes to mask DNA), mechanical (electric shock opens pores in PM, micro injection, bombardment with DNA)
|
|
|
What are markers used for in recombinant DNA?
|
They are used to see which cells have uptaken and expressed the gene in their genome
|
|
|
What are knockout mice?
|
Mice with selective gene inactivation
|
|
|
How are knockout mice made?
|
Gene of interest is cloned into a vector and a segment of DNA is added to inactivate it
This gene is added to the mouse embryonic stem cells Homologous recombination occurs to switch out the functional gene Cells are screened for the gene and knocked out cells are injected into the embryo |
|
|
What is the role of antisense RNA in gene expression?
|
Antisense RNA is complementary to mRNA and binds to it so that translation is inhibited
|
|
|
What is the role of siRNA in gene expression?
|
siRNA binds to mRNA at a spot, catalysing it's breakdown so that it cannot be translated.
|
|
|
What is the difference between a genomic library and a cDNA library?
|
A gene (genomic) library represents all genes
of an organism cDNA libraries represents only those genes being expressed when the RNA was obtained from the cells. |
|
|
Why is diagnostic DNA testing useful in a clinical setting?
|
It allows the diagnosis of conditions early, making early intervention possible
|
|
|
What are some ethical issues which may arise when people recieve the results of DNA tests
|
Things like abortion: The right to life vs the right to choose. When is this legal?
What do people do with the information? Who do they share it with? |
|
|
What are the 3 different ways of detecting genetic variations?
|
Allele specific cleavage
Allele specific oligonucleotide hybridisation Allele specific PCR |
|
|
What is allele specific cleavage?
|
A means of detecting an exact mutation in a genome
-The exact mutation must be known -Restriction enzymes are used to cut at the gene loci, determining whether or not a mutation is present -Differences show up in gel electrophoresis |
|
|
What is allele specific oligonucleotide hybridisation?
|
A means of detecting known sequence changes in genomes.
Labelled oligonucleotides are used to hybridise to the normal or mutant gene- Act as a probe |
|
|
What is allele specific PCR?
|
A means of detecting mutations on primer DNA
-2 different types of oligonucleotide primers are mixed in the the DNA to detect both normal and mutant alleles. -The DNA that is amplified determines the presence of an allele |
|
|
What is DNA fingerprinting?
|
The examination of sequences such as STR’s or SNP’s, which are highly variable between human beings, to provide each individual with a unique genetic identity.
|
|
|
What is an SNP?
|
Single nucleotide polymorphism
|
|
|
What is an STP?
|
Short tandem repeats
|
|
|
What are the steps of cloning proteins for disease treatment?
|
1) Isolate the gene
2) Introduce the gene to a host which will express it 3) Grow in host cells on a commercial scale 4) Purify to meed standards 5) Clinical testing 6) Clinical approval |
|
|
What are some examples of protein production for gene treatment?
|
Tissue Plasminogen Activator - Produces plasminogen, a clot dissolver
Human Growth Hormone- Used to treat dwarfism Hep B virus |
|
|
What is a DNA vaccine?
|
Directly inject or fire DNA coated gold beads into muscle
|
|
|
How are more effective vaccines made?
|
-Microbial DNA is extracted and cloned into plasmid vectors and these are inserted into mouse embryonic cells.
-The mice are grouped according to which plasmid they received and are infected with the microbe. -Surviving mice are put in smaller and smaller groups until the plasmid which confers immunity is isolated. -This gene can then be used to create a vaccine for humans. |
|
|
What are plant vaccines?
|
DNA that is introduced into a plant to act as a vaccination upon ingestion
|
|
|
How do plant vaccines work?
|
Antigen genes are isolated from pathogens such as measles and hepatitis, cloned into vectors and introduced to plant cells.
The antigens will be produced in plant tissues, and eating the plant will induce production of antibodies, conferring immunity. |
|
|
What are the advantages of plant vaccines?
|
They are thermally stable and widely available
|
|
|
What are the disadvantages of traditional vaccine methods?
|
Short lasting
Can be infective Allergic response is possible Less temperature stable |
|
|
What are the advantages of DNA vaccines?
|
Single dose
Stable Cheaper Easy to administrate Not infections/replicative |
|
|
What is pharming?
|
The use of animals as bioreactors for protein production
|
|
|
How do Activator proteins regulate gene expression?
|
They initiate transcription by binding directly to DNA
-There are various different types -Partially complementary regions to bind to DNA |
|
|
What is differential transcription?
|
The difference of protein production patterns between different cell types
|
|
|
What is the role of non-DNA binding proteins in gene expression
|
They bind to DNA bound proteins, regulating the shape and activity of those proteins though many mechanisms
-phosphorylation etc |
|
|
How do repressors regulate gene expression?
|
They compete with activators, mask activator effects or negatively interact with the transcription initiator complex at the promoter region
|
|
|
How does chromatin remodelling influence gene expression?
|
It uses ATP to alter the level of packaging it is in.
-Higher order packaging=silenced gene -Lower order packaging=expressible |
|
|
How do histone extensions help regulate gene expression?
|
They have N-terminals which have lots of amino acids so that they are more able to interact with proteins
|
|
|
How do barrier sequences regulate gene expression?
|
They physically inhibit expression by
-anchoring DNA to fixed sites -erasing markers which are required for gene activation |
|
|
How does acetylation regulate gene expression?
|
The 4 core histone proteins can have acetyl groups added to them.
Hypoacetylation=inactivation Hyperacetylation=activation |
|
|
What is DNA methylation?
|
The reversible addition of a methyl group at the 5' position of cytosine which DOES NOT affect DNA sequence.
|
|
|
What is an epigenetic alteration?
|
A heritable genetic alteration which does not involve the genetic seuence
|
|
|
What are the enzymes which control methylation?
|
Methylase and demethylase
So creative guys... |
|
|
How can methylation regulate gene expression?
|
Methylation sites are found typically in clusters around the promotor region. Methyl C binding proteins bind tightly to repress transcription.
|
|
|
When may diseases arise due to methylation?
|
When mutations occur in the genes controlling printing or when genes which control activator/repressor proteins are methylated
|
|
|
What do hypomethylation and hypomethyation lead to?
|
Hypomethylation-Exaggerated gene expression (can cause instability)
Hypermethylation- Repressed expression (can lead to inactivation of important genes) |
|
|
How does X Chromosome inactivation work?
|
The inactive X has the 'xist' gene
-xist gene is methylated and transcriptionally active -RNA is transcribed from this gene and binds to the same chromosome that transcribed it, inactivating it |
|
|
Where does cell differentiation arise from?
|
Differential gene expression which causes cells to become more specialised
|
|
|
What is a clone?
|
Something that is genetically identical to its original cell source
|
|
|
How is a clone made?
|
-Cells are extracted from an organism
-The cell is fused with an enucleated egg using eletcric pulses to mirror fertilisation so embryonic development will occur -Early embryos are transferred into surrogate mothers |
|
|
What are some issues with the cloning process?
|
Inefficient
It is difficult to produce clones, the older an organism is |
|
|
How can embryonic stem cells be harvested?
|
Extracted from an early embryo and cultured.
-They can also be induced to differentiate (theoretically) |
|
|
What is theraputic cloning?
|
Where ESCs can be customised for particular patients by using their own genome to produce genetically identical stem cells in order to reduce the risk of immune rejection
Can also be used in other organisms to do the same thing ie)transgenic pigs and organ transfer |
|
|
What is Somatic Cell Nuclear Transfer
|
The initial stage of cloning, where one genome is transferred into an egg for the growth of ESCs
|
|
|
Where is SCNT legal in australia? What are the boundaries surrounding this?
|
In NSW and VIC.
-Embryo can't develop for longer than 14 days -Embryo cannot be implanted -Sperms and eggs can't be used to produce ESCs |
|
|
What are some issues surrounding the feasibility of SCNT?
|
efficiency
Limited eggs mtDNA means there isn't a perfect math ESCs can be contaminated by the cultures that they grow in Purification of the ESCs is paramount to ensure teratomas don't develop Ethics |
|
|
What are adult stem cells?
|
multipotent stem cell lines found in tissues which are involved in repair and replenishment
|
|
|
What are the issues surrounding the used of ASCs in the lab?
|
Few in number in the body
Hard to culture |
|
|
How are ASCs distinguished?
|
By their behaviour, not by their appearance
|
|
|
How are induced pluripotent cells produced?
|
ASCs are extracted and cultured
4 Transcription factors are added to bring them back to a pluripotent state |
|
|
How can iPSCs be usedto treat disease?
|
By cloning a genome with a defect, the progression of a disease at a cellular level can be determined
-This information can be used to work out treatments |
|
|
What are questions surrounding the efficacy of iPSCs
|
How do epigenetic differences 9methylation and telomere lengths) affect the quality of the cells
What is the impact of pre-existing genetic abnormalities from the ASC |
|
|
What is human gene therapy?
|
The ADDITION of new genes into somatic cells internally or externally to the body
|
|
|
What are receptors?
|
Intra/extracellular recognition mechanisms which respond to certain stimuli
-Signals bind, triggering biological response -Proteins |
|
|
What is receptor saturation?
|
When a cell is fully stimulated and no additional response can occur
|
|
|
What are the fates of cells responding to receptors?
|
Survive
Grow and divide Differentiate Gene expression Die |
|
|
What are 2 types of intercellular signalling?
|
Signalling with secreted molecules
Signalling with plasma membrane bout molecules |
|
|
What are the types of cell receptors?
|
Cell surface receptors
Cytoplasmic receptors |
|
|
What are the 3 different types of hormone communication?
|
Endocrine secretion
Autocride secretion Paracrine Secretion |
|
|
What is endocrine secretion?
|
Hormones are released into the bloodstream where they will travel to target cells which have the hormone-specific receptor
|
|
|
How are endocrine systems usually controlled?
|
By feedback signals which come from the target cells to keep a balance of product in place
|
|
|
Which centre controls many hormone release pathways?
|
The pituitary gland
|
|
|
What is autocrine secretion?
|
A hormone which is released and responded to by the same cell via diffusion.
|
|
|
When is autocrine secretion at its strongest?
|
When a large group of identical celles are clustered together, secreting the same hormone
-Concentration of the signal is higher |
|
|
What is paracrine signalling?
|
When a hormone targets neighbouring cells
|
|
|
Between which cells to synapses occur?
|
Muscle-nerve
nerve-nerve |
|
|
What is a synapse?
|
A gap between signalling and target cells
|
|
|
Are neurotransmitters multi-purpose?
|
Yes. Neurons can use the same neurotransmitter to communicate with different targets
|
|
|
What is a neuromuscular junction?
|
The synapse between a muscle and a nerve
|
|
|
What are the components of the synapse?
|
Axon terminal
Post-synaptic terminal Synaptic cleft |
|
|
What is the axon terminal?
|
The presynaptic cell
-contains synaptic vesicles carrying neurotransmitter -Mitochondria |
|
|
What is the synaptic cleft?
|
The gap (20-50nm) between the cells
|
|
|
What is the difference between the synapses of smooth and skeletal muscle
|
They have the same release mechanism, but there is no 'synapse' as such in the smooth muscle.
The nerve has swellings which release the NTM |
|
|
What is a variscosity?
|
A bulge in the nerve which releases NTM to smooth muscle
|
|
|
Describe the communication via gap junctions
|
An electrical and metabolic communication which occurs very fast
|
|
|
Describe a signal
|
A stimulus or chemical signal allows cells to process and respond to information. They can be local or distant and to interpret a signal a cell must have the appropriate receptor for it
|
|
|
What is a signal transdcution pathway?
|
The whole signalling process from detection to the final response
-Signal -Receptor -Transduction -Effects |
|
|
What is a ligand?
|
Signalling molecules that remporarily bind to receptors
|
|
|
How does the ligand influence the receptor when it binds to it?
|
it changes shape
|
|
|
How do inhibitors (receptor) work?
|
They bind to the ligand binding sites, blocking the ligand from binding.
They can also bind themselves to the ligand to prevent them rom binding to the receptor |
|
|
What are ion channel receptors?
|
Channels that act as gates, restricting ion movement.
-Respond quickly -Passive -Respond to specific ligands to open/close |
|
|
What is a protein kinase?
|
An enzyme which phosphorylates other molecules
-When activated, some receptor proteins become kinases |
|
|
What is autophosphorylation
|
What a kinase phosphorylates itself.
|
|
|
In terms of insulin and the signalling pathway, how do kinases work?
|
-Insulin binds to the receptor
-Receptor dimerizes, exposing an active site in the cytoplasm -Receptors phosphorylate the other -Insulin response substrate is phosphorylated and activated, goes on to amplify and message |
|
|
What are g-proteins?
|
A class of 7 g-protein linked receptors
|
|
|
How do g-proteins work, in terms of signalling?
|
-Ligand binds to the extracellular site, changing the protein shape on the cytoplasm side
-This exposes the g-protein binding site -GTP bound subunit detaches and moves along the membrane to find other effector proteins to amplify the message |
|
|
How do cytoplasmic receptors function?
|
-Ligand binds to receptor located in cytoplasm
-Can enter the nucleus and act as a transcrption factor |
|
|
What are intracellular signalling molecules?
|
Molecules that mediate between the receptor and cellular response by transmitting and amplifying the message throughout the cell to produce a specific response.
-secondary messengers and signalling proteins |
|
|
Describe the RAS protein cascade
|
-receptor dimerizes and phosphorylates when it encounters its ligand
-Adaptor protein becomes active and activates RAS -RAS is stimulated to swat ADP for ATP -RAS activates more protein kinases -MAPK acts as a transcription factor and stimulates cellular replication |
|
|
Why are steps in cell signalling so tightly regulated?
|
So that they can avoid overactivity, which leads to disease
|
|
|
Why are a variety of steps in the signalling pathway ideal?
|
Because they allow a variety of responses due to the different pathways available
|
|
|
What are the components of the central nervous system?
|
cerebrum
cerebellum brainstem spinal cord |
|
|
What are the 2 branches of the PNS?
|
Somatic and autonomic
|
|
|
Which of the 3 germ layers gives rise to neural tissue?
|
ectoderm-from the neural plate/notochrod
|
|
|
What is the process of neurulation?
|
The development of the neural tube from the neural plate
-Notochord induces the neural plate to fold -Neural plate fuses at the dorsal margins -Nerual crest arises at the point of fusion PLATE GROOVE FOLDS TUBE |
|
|
What is the anterior neuropore
|
The rostral neuropore. The gap at the top of the neural tube during development
|
|
|
What is the caudal neuropore?
|
The posterior neuropore at the base of the neural tube during develpoment
|

