![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
23 Cards in this Set
- Front
- Back
|
Which nuclei in the thalamus are important for peripheral sensory function? |
VPL, VPM, DPM (dorsal medial), CM (intralaminar centromedian), PF (parafascicular) |
|
|
What can the ascending anterior lateral spinothalamic tract be functionally divided into?
What does each encode? |
Paleo-spinothalamic pathway (emotional and visceral responses to pain, medial affective-motivation, influences descending pathways from brain stem that modulate pain) Neo-spinothalamic pathway (intensity, location, and quality of pain, lateral sensory-discriminative, sharp, well-localized, relayed rapidly to somatosensory cortex) |
|
|
The VPL receives both protopathic (nociceptive) and epicritic sensory information from the _____ and _____ while the VPM receives similar sensory information from the _____. Regarding pain, VPL and VPM receive pain fibers from the ‘___-spinothalamic tract that is relayed on to the Lateral Pain System. The DPM, CM and PF nuclei receive pain information from the _____-spinothalamic tract and relay this on to the Medial Pain System. |
The VPL receives both protopathic (nociceptive) and epicritic sensory information from the limbs and body while the VPM receives similar sensory information from the face. Regarding pain, VPL and VPM receive pain fibers from the ‘neo-spinothalamic tract that is relayed on to the Lateral Pain System. The DPM, CM and PF nuclei receive pain information from the paleo-spinothalamic tract and relay this on to the Medial Pain System. |
|
|
What are the components of the lateral pain system?
Medial pain system?
|
Primary and secondary somatosensory cortex
Anterior cingulate, ínsula, amygdala, hypothalamus |
|
|
What three stimuli do free nerve endings detect? |
Temperature Mechanical Chemical
|
|
|
What temperature receptor potential channels (TRP) are sensitive to >43 degrees and capsaicin?
Which sensitive to <25 degrees? |
TRPV1 TRPM8 |
|
|
What two fiber types conduct pain? |
A-delta C-fibers |
|
|
Which fibers are thinly myelinated and carry temperature, mechanical pain, discrete location, fast, and sharp pain sensory information? |
A-delta fibers |
|
|
Which fibers are unmyelinated, transmit temperature, mechanical, and chemical pain (polymodal), diffuse, and slow pain? |
C-fibers |
|
|
Where do the cell bodies of A-delta and C fibers lie? What three neurotransmitters are used? |
Dorsal root ganglia Glutamate, substance P, CGRP |
|
|
1. Peripheral Pain Receptor Sensitization ̧Stimulation (tissue injury) of nociceptive receptors triggers release of several substances (H+ ion, 5-HT, ATP, _____, _____) that activate free nerve endings to fire an action potential (AP) back to the spinal cord dorsal horn
̧2. Activation of the nociceptive receptors also causes the local (peripheral) release of substance __ and _____ from the free nerve endings at the site of injury. Substance P and CGRP in turn cause release of ____ from mast cells and vasodilation of local blood vessels.
̧3. The combination of local tissue injury (inflammation) with release of the above substances and the release of substance P and CGRP from the free nerve endings sensitizes the free nerve ending receptors such that their threshold for activation is ______
̧4. Finally, the inflammatory chemical milieu activates previously _____ nociceptive receptors on free nerve endings to become active thereby increasing the temporal and spatial _____ of APs traveling to the dorsal horn |
1. Peripheral Pain Receptor Sensitization ̧Stimulation (tissue injury) of nociceptive receptors triggers release of several substances (H+ ion, 5-HT, ATP, bradykinin, prostaglandins) that activate free nerve endings to fire an action potential (AP) back to the spinal cord dorsal horn
̧2. Activation of the nociceptive receptors also causes the local (peripheral) release of substance P and CGRP from the free nerve endings at the site of injury. Substance P and CGRP in turn cause release of histamine from mast cells and vasodilation of local blood vessels.
̧3. The combination of local tissue injury (inflammation) with release of the above substances and the release of substance P and CGRP from the free nerve endings sensitizes the free nerve ending receptors such that their threshold for activation is lowered
̧4. Finally, the inflammatory chemical milieu activates previously silent nociceptive receptors on free nerve endings to become active thereby increasing the temporal and spatial summation of APs traveling to the dorsal horn |
|
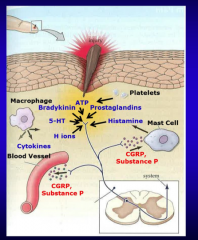
Review of peripheral pain sensitization |

|
|
|
What are neurons in the laminae I and II of the dorsal horn that respond only to A-delta or C fiber APs and encode only pain?
|
Nociceptive specific neurons (SPNs) |
|
|
What are neurons in laminae V of the dorsal horn that respond to a variety of synaptic input encode pain and non-pain stimuli?
How do they fire APs? In other words, the greater the C-fiber AP frequency, the greater the AP response in the _________.
What are WDRNs responsible for? |

|
|
|
Describe the process of WIND UP (signal amplification). => How do repetitive APs from C-fibers trigger wind up? |
Glutamate activation of WDRN AMPA receptors and CGRP activation of WDRN CGRP receptors => WDRN depolarization => Mg2+ block of NMDA channel removed => insertion of more Na+ channels and blockade of K+ channels in WDRNs |
|
|
How does substance P contribute to the process of WIND UP (signal amplification)?
What does the combined action of these neurotransmitters lead to a lower threshold for firing APs? |
Activates NK1 receptors => prolonging of WDRN depolarization
Combined activation => reduces WDRN threshold => increase insertion of more receptors in WDRN => lower threshold for firing APs |
|
|
What is the functional consequence of WIND UP? |
Brief c-fiber stimulation => long lasting facilitation of the pain pathway stimulated => hyperalgesia and chronic pain |
|
|
Define gate-control mechanisms: |
A-beta fibers => dorsal column interneurons => inhibit WDR neurons => blunts activation of WDR neurons in response to A-delta and C-fiber activity. |
|
|
Descending pathways: Cortex, amygdala, hypothalamus all impinge upon periaqueductal _____ and reticular formation neurons that in turn send descending fibers to modulate lamina II neurons in the ______ horn. These descending systems may either inhibit or facilitate pain. |
Cortex, amygdala, hypothalamus all impinge upon periaqueductal gray and reticular formation neurons that in turn send descending fibers to modulate lamina II neurons in the dorsal horn. These descending systems may either inhibit or facilitate pain. |
|
|
Endogenous opioid: Describe it! |
Opioid receptor activation => block pre-synaptic voltage gated Ca2+ channel and/or opening or K+ channels => hyper polarizing postsynaptic neuron => reduce APs |
|
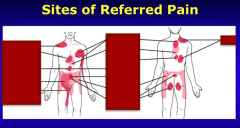
Identify the sites of referred pain: Liver, SI, appendix, right ureter, heart, stomach, gallbladder, ovary, colon, kidney, bladder |

|
|
|
The CNS pathways responsible for conducting visceral pain have recently been modified to account for a newly discovered pathway. Nociceptive receptors lying in the visceral (intrathoracic, abdominal, pelvic organs) until recently were thought to synapse on dorsal horn neurons primarily devoted to surface (dermatomal) receptors and that viscera were sparsely populated by these receptors. The latter statement remains correct and there is significant sharing of visceral nociceptive activity with the dermatomal system. In fact, this biological arrangement is thought to be the basis of referred pain. For example, poorly localized nociceptive receptors in heart muscle may generate pain localized not only to the midline-left chest but also to the C8-T1 dermatome (see above figure). Heart muscle pain may also be referred into the neck region (not shown in above figure). |
The CNS pathways responsible for conducting visceral pain have recently been modified to account for a newly discovered pathway. Nociceptive receptors lying in the visceral (intrathoracic, abdominal, pelvic organs) until recently were thought to synapse on dorsal horn neurons primarily devoted to surface (dermatomal) receptors and that viscera were sparsely populated by these receptors. The latter statement remains correct and there is significant sharing of visceral nociceptive activity with the dermatomal system. In fact, this biological arrangement is thought to be the basis of referred pain. For example, poorly localized nociceptive receptors in heart muscle may generate pain localized not only to the midline-left chest but also to the C8-T1 dermatome (see above figure). Heart muscle pain may also be referred into the neck region (not shown in above figure). |
|
|
Draw out the visceral pain pathway.
|

|

