![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
99 Cards in this Set
- Front
- Back
|
What are the 3 ways of representing a carbohydrate? |
Fischer Projection Haworth Projection Chair Conformation |
|
|
What are the 2 major subgroups of isomers? |
Stereoisomers and constitutional isomers |
|
|
What are the 2 subgroups of a stereoisomer? |
1) Enantiomer 2) Diastereomer |
|
|
What's the difference between an enantiomer and a diastereomer? |
Enantiomers are nonsuperimposable mirror images while diastereomers are not mirror images at all |
|
|
What are the 2 subgroups of diastereomers? |
1) Epimers 2) Anomers |
|
|
What is an anomer and give an example |
An isomer that differ at a new asymmetric carbon atom after ring closure Ex: alpha va beta D-glucose |
|
|
How does the stereochemistry of an enantiomer effect its interaction with an enzyme? |
Only one enantiomer will be a substrate for an enzyme, not both forms |
|
|
What are the 2 families of sugars based on structure and describe their structures? |
1) Aldoses have a terminal aldehyde group 2) Ketoses have an intrachain ketone group |
|
|
How do we distinguish between L and R isomers of in a fischer projection assuming that the methanol is at the bottom? |
Hydroxyl attached to chiral carbon that is farthest from the aldehyde/keto group is on the left for any L isomer or on the right for any D isomer
|
|
|
How do we mathematically determine the possible number of isomers in a sugar?
How does this differ for a ketose and aldose? |
2^n where n = number of chiral centers
Ketose always has 1 less chiral center due to planar ketone |
|
|
What's the name for a 3, 4 ,5, and 6 carbon sugar? |
Triose, Tetrose, Pentose, and Hexose |
|
|
Describe the structure of Glyceraldehyde and draw it in its D form |
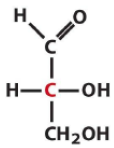
Triose Aldose
|
|
|
Describe the structure of Erythrose and draw it in it's D form |
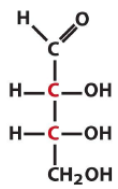
Tetrose Aldose with all the hydroxyls on the same side |
|
|
Describe the structure of Ribose and draw it in it's D form |
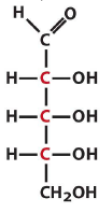
Pentose Aldose with all the hydroxyls on the same side |
|
|
Describe the structure of Arabinose and draw it in it's D form |
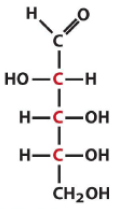
Pentose Aldose with the C2 hydroxyl being opposite of the rest |
|
|
Describe the structure of Xylose and draw it in it's D form |
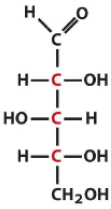
Pentose Aldose with the C3 hydroxyl being opposite of the rest |
|
|
Describe the cyclization of Ribose and then draw it. |
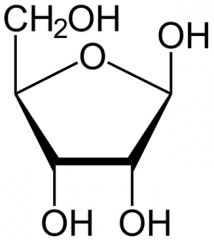
It's a furanose (5 membered ring) The carbonyl on C1 becomes an alcohol as the Hydroxyl on C4 becomes an ether, leaving the C5 methanol attached to the C4 |
|
|
Describe the structure of Glucose and draw it in it's D form |
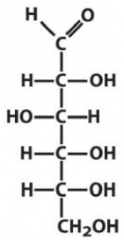
Aldose Hexose with the C3 hydroxyl on the opposite of the rest |
|
|
Describe the structure of Fructose and draw it in it's D form |
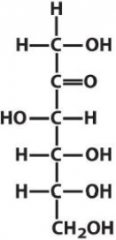
Ketone Hexose with the C3 hydroxyl being opposite of the rest and methanols on both terminals Ketone on C2 |
|
|
What's the relationship between Mannose, Glucose, and Galactose and describe their structural relationship |
Mannose and Galactose are epimers of glucose Mannose is a C2 epimer Galactose is a C4 epimer |
|
|
Describe the structure of Mannose and draw it in it's D form |
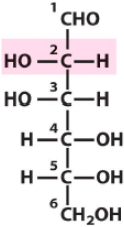
Aldose Hexose with C2 and C3 hydroxyls being opposite of C4 and C5 hydroxyls |
|
|
Describe the structure of Galactose and draw it in it's D form |
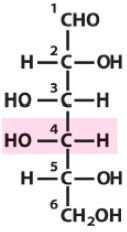
Aldose Hexose with C2 and C5 hydroxyls being opposite of C3 and C4 hydroxyls |
|
|
What is a hemiacetyl and what are the reactants in the reaction? |
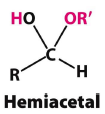
Formed from an aldehyde and an alcohol |
|
|
What's the difference between pyran and furan? |
Pyran is a 6 membered ring while Furan is a aromatic 5 membered ring |
|
|
Pyranose can take 2 forms, how do they differ? Include Haworth and Chair conformation in description |
The anomeric carbon's (C1) hydroxyl can be Up or Down on a Haworth or Axial/Equitorial for chairs |
|
|
When does a hemiacetal form? |
After the C5 OH attacks the C1 aldehyde groups carbonyl |
|
|
Why is the Beta-D anomeric form of glycopyranose predominate over the Alpha-D form? |
The Beta-D is more stable due to less steric hinderance between the C1 and C2 hydroxyls since they're oriented in opposite direction |
|
|
What's a hemiketal and under what conditions does it form? |
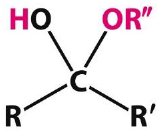
Forms after C5 OH attacks the C2 carbonyl Ketone
|
|
|
What is the major heat-sensitive sugar in honey? |
Fructopyranose |
|
|
Hexose carbohydrates can exist in 2 conformation, what are they? |
Chair and Boat conformation |
|
|
How can copper aid in the detection of glucose? |
The aldehyde group in open chain glucose can reduce copper which results in a visible color change |
|
|
What glycosylated hemoglobin do we look for when identifying diabetics? |
Hemoglobin A1c |
|
|
How does hemoglobin A1c form? |
The open chain glucose's aldehyde group reacts with a hemoglobins amine group leading to a schiff base which rearranges to an Amadori product which is the hemoglobin A1c |
|
|
Glycosidic bonds form between the _____ carbon of a monosaccharide to ___, ____, and ____ atoms of other molecules |
Anomeric, N, S, O |
|
|
True or false: Monosaccharides can be modified at other than the anomeric carbon? |
True |
|
|
What are the 3 common disaccharides discussed in lecture? |
Maltose, Lactose, and Sucrose |
|
|
Describe maltose |
Maltose is a repeating unit of amylose |
|
|
Which sugar discussed in lecture is "non-reducing?" |
Sucrose |
|
|
True or False: |
True |
|
|
What is the main storage polysaccharide in plants and what are they composed of? |
Starch is a mixture of amylose (10-20%) and amylopectin (80-90%) |
|
|
Compare and contract the structure of amylose and amylopectin |
Amylopectin has an alpha-1,6 glucose branch every 30 residues Both Amylose and Amylopectin have alpha-1,4 glucose links
|
|
|
Describe the structure of the animal storage product glycogen. |
Glucose chains joined by alpha-1,4 links Every 10 residues there's an alpha-1,6 residue |
|
|
Descrive the structure of the most abundant natural polymer in the world, cellulose |
Beta-1,4 D-glucose polymer |
|
|
What about cellulose's secondary structure makes it indigestible to animals? |
Hydrogen bonding allows for the formation of sheets which are harder to break down than helices |
|
|
What is it about glycogens structure that makes it better as a storage unit? |
The occasional (every 10 sugars) alpha-1,6 gives glycogen a less linear shape (more globular?) which provides more ends for rapid degradation compared to a straight chain |
|
|
What amino acids link to carbohydrates? |
Asparagine, Serine, and Threonine |
|
|
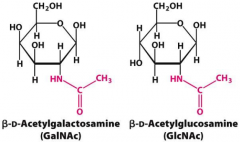
Compare and contract GlcNAc and GalNAc |
GlcNAc is C2 N-linked to an aspragine GalNAc is C2 O-linked to a threonine
The C4 hydroxyl are opposite in each structure |
|
|
Compare the sugar content of glycoproteins to that of proteoglycans and to Mucins |
Glycoproteins are 1-10% carbohydrate at most Proteoglycan are 80-95% carbohydrate Mucins are also mostly carbohydrate |
|
|
Where are glycoproteins mostly found? |
Cell membrane proteins and secreted proteins |
|
|
What is the function of the carbohydate in a glycoprotein? |
Acts as a trafficking tag |
|
|
Where are proteoglycans mostly found? |
Structural components such as cartilage |
|
|
What is the predominant carbohydrate found in mucins? |
GalNAc |
|
|
Where are Mucins mostly found? |
Found in mucus and other lubricants |
|
|
What are the 6 functions of protein glycosylation discussed in lecture? |
1) To increase the complexity of the proteome 2) To direct trafficking 3) To direction folding (or lack of folding) 4) To contribute structural strength 5) To Provide protection against proteases 6) Involved in receptor binding |
|
|
In what part of the cell does glycosylation occur? |
ONLY in the ER Lumen and Golgi NOT Cytoplasm |
|
|
What would prevent a glycosylated protein from being secreted? |
1) A mutation in a specific glycosyltransferase preventing it from getting to the ER 2) A mutation in the secretion apparatus that prevents all proteins from being secreted |
|
|
What do all N-linked sugars have in common? |
A (5) pentasaccharide core composed of 2 GlcNAc's and 3 Mannoses All N-linked sugars can only bind to an Asn, if it's in the context of Asn-X-Thr or Asn-X-Ser where X cannot be proline |
|
|
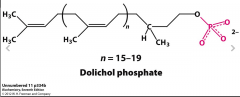
What specialized lipid are N-linked oligosaccharides assembled on? |
Dolichol Phosphate |
|
|
What is the function of Dolichol Phosphate? How does GlcNAc play a role? |
Act as a scaffold that GlcNAc binds to first at which point a variety of sugars can be added |
|
|
Draw the structure of Dolichol Phosphate |
|
|
|
How do we start a dolichol-phosphate oligosaccharide? |
GlCNAc-1-P Transferase adds UDP-GlcNAc at the expense of 1 phophate Thus, the original dolichol phosphate is then bound to a GlCNAc phosphate Product: Dolichol-P-P-GlcNAc |
|
|
What is the precursor used in the biosynthesis of oligosaccharides destined to become protein modification? |
UDP-glucose |
|
|
What enzyme do we use to transfer sugars? |
Glycosyltransferase |
|
|
What happens to a dolichol-phosphate oligosaccharide once it is completely assembled? |
It is tranferred to an Asn residue on the target protein that is to be glycosylated |
|
|
How is ABO blood type determined? |
It is determined by the presence of 2 extra glycosyltransferases on top of the enzyme needed to synthesize the O antigen since everyone has that |
|
|
What enzyme is responsible for the A antigen in blood types? |
GalNac transferase adds the extra N-acetylgalatosamine seen in people expressing A blood types |
|
|
What enzyme is responsible for the B antigen in blood types? |
Gal Transferase adds the extra galactose |
|
|
What do all blood types have in common? |
A O-antigen backbone composed of: R - Galactose - Glucose - Galactose - Fucose |
|
|
Why do we have different blood types in the human gene pool? |
Pathogens mimic cell surface proteins as an evasion tactic. Having multiple glycosylation patterns makes it harder for them which increases our resistance |
|
|
Who is the universal donor and who is the universal acceptor? |
Universal Donor = O Universal Acceptor = AB |
|
|
What is the function of Hyaluronan? |
To provide a backbone for aggrecan to bind through it's 1st globular domain |
|
|
What is a glycoconjugate? |
A polysaccharide linked to a protein or peptide |
|
|
What are the distinguishing compositional feature of Proteoglycans? |
It is composed of a few proteins lined with lots of glycosaminoglycans where at least 1 of the 2 sugars in the glycosaminoglycan unit are a negatively charged carboxylate or sulfate
|
|
|
What is a glycosaminoglycan? |
A long unbranched polysaccharides consisting of a repeating disaccharide unit |
|
|
What is a heteropolysaccharide? |
A glycosaminoglycan complex carb. formed by combining a carb. with a non-carb. or carb-derivative group where at least 1 of the 2 sugars in the glycosaminoglycan unit are a negatively charged carboxylate or sulfate |
|
|
What examples of proteoglycans were discussed in lecture? |
Chondroitin 6-sulfate Keratan Sulfate Heparin |
|
|
What examples of Heteropolysaccharides were discussed in lecture? |
Dermatan Sulfate Hyaluronate |
|
|
What allows for heteropolysaccharides and proteoglycans to exist in aqueous solution? |
Carboxyl groups, sulfates, or uronic acid |
|
|
Describe the structure of chitin and it's function |
It is a long polymer of GlcNAc mostly found in exoskeletons |
|
|
Describe the backbone and overall structure of a Mucin |
Backbone: It has VNTR's (Variable number tanden repeat regions) with a high degree of serine and threonine O-glycosylation through GalNAc Overall highly branched and extended variable structure due to the high glycosylation |
|
|
What is the function of erythropoietin? |
It stimulates production of RBC's |
|
|
What is the purpose of glycosylating EPO? |
Glycosylation enhaces the stability of the protein in the blood |
|
|
What is the function of recombinant EPO in sports? How do we identify recombinant EPO? |
Recombinant EPO can be used to increase oxygen-carrying capacity in endurance athletes. Labs can identify recombinant EPO through isoelectric focusing to differentiate glycosylation patterns between native and recombinant EPO |
|
|
What are lectins? |
Proteins that bind to specfic oligosaccharides aka Glycan-binding proteins |
|
|
What are the 2 types of lectins discussed in lecture? |
L (lymph) lectin C (calcium-requiring) lectin |
|
|
Where are L-lectins found and what is their proposed function? |
Found in leguminous plants and in the eukaryotic ER maybe an insectiside in plants or chaperone protein in the ER |
|
|
What is unique about C-type lectins? |
They have a calcium binding domain that acts as a bridge between the protein and the sugar |
|
|
What protein in the C-type lectin family is involved in the inflammatory response? What does it do? |
Selectins bind immune-system cells to sites of injury in the inflammatory response |
|
|
What protein in the C-type lectin family is involved in reproduction? |
L-selectins are produced by embryos when they are ready to attach to the endometrium of the mothers uterus |
|
|
What are the 3 types of selectins and where do they bind? |
L (lympth carbs) E (endothelial carbs ) P (Platelet carbs) |
|
|
What would prevent endothelial cells from adhering to leukocytes? |
1) Lack of calcium in the medium which inhibits C-type lectins 2) Glycosidases that cleave extracellular carb structures between neighboring cells 3) medium is hot? |
|
|
What occurs in I-cell disease at the molecular level? |
Lysosomes containg large inclusions of undigested glycosaminoglycans and glycolipids |
|
|
What causes I-cell disease? |
Improper M6P glycosylation of enzymes needed to degrade glycosaminoglycans and glycolipids
Enzymes are exported to blood and urine instead of the lysosome |
|
|
How does the influenza virus invade cells? |
Virus recognizes sialic acid residues linked to galactose on cell-surface glycoproteins
The virus binds via hemagglutinin and is endocytosed |
|
|
What is the function of Neuraminidase in the spread of the new influenza virions? |
To exit the cell, the virions binds to extracellular hemagglutinin. Neuraminidase then cleaves the glycosidic bond to the sialic residues and allows the virions to spread |
|
|
How do influenza medications work? |
Influenza medication inhibit the action of neuraminidase through transition-state analog inhibition which prevents spread of new virions |
|
|
What could be responsible for influenza resistance? |
1) Reduced extracellular sialic acid levels 2) Hemagglutinin binding antibodies 3) Neuraminidase-blocking activity |
|
|
What are the 2 ways to inhbit neuraminidase |
1) Transition state analog inhibition 2) Hydrolysis, phosphorylation, or glycosylation of neuraminidase |
|
|
What makes a sugar reducing vs non-reducing? |
An unbound anomeric carbon can exist in open-chain form, allowing for aldehydic activity. Non-reducing sugars are those that have both of their anomeric carbons bound and thus have no aldehydic activity |

