![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
18 Cards in this Set
- Front
- Back
|
Regions of high electron density act as...
|
nucleophiles
|
|
|
T/F Resonance outweighs inductive effect
|
True
|
|
|
An electronegative atom withdraws electron density, increasing/decreasing acidity? Electrophilicity? Basicity? Nucleophilicity?
|
Increases the acidity, increases the electrophilicity, and decreasing the basicity, and decreasing the nucleophilicity.
|
|
|
Relative electronegativity of common atoms in organic chemistry
|
Fonclbrisch
|
|
|
Alkyl groups are electron donating or electron withdrawing?
|
Electron donating
|
|
|
Huckel rule
|
4n + 2 pi electrons in cyclic pi network
|
|
|
Electron withdrawing groups make an acid ...
|
stronger
|
|
|
An electron donating group makes an acid ...
|
weaker
|
|
|
Electron donating groups make a base ..
|
stronger
|
|
|
Electron withdraw groups make a base ...
|
weaker
|
|
|
5-10-15-20
|
pKa's: carboxylic acid, phenol, alcohol, carbonyl alpha proton
|
|
|
Acid strength factors: size
|
For comparing acids involving atoms within the same column
Larger atom = longer bond = stronger acid |
|
|
Acid strength factors: electronegativity
|
For comparing acids involving atoms of the same column
The more electronegative the atom, the stronger the acid The less electronegative the atom, the stronger the base |
|
|
Acid strength factors: hybridization
|
The more s character, the stronger the acid due to the bond being able to be cleaved heterolytically more easily
|
|
|
Which is more of a significant secondary factor, resonance or induction?
|
Resonance
|
|
|
Henderson-Hasselbalch equation:
|

|
|
|
Ka equation
|
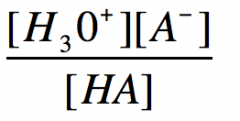
|
|
|
rate constant
|

|

