![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
170 Cards in this Set
- Front
- Back
|
allylic group
|
group on a C adjacent to a db
|
|
|
benzylic group
|
group on a C adjacent to a benzene ring or substituted benzene ring
|
|
|
many cases, allylic & benzylic groups are
|
unusually reactive
|
|
|
allylic carbocations are
|
resonance stabilized
|
|
|
allyl cation
|

resonance stabilized
|
|
|
resonance stabilization of the benzyl cation
|

|
|
|
resonance structures of the benzyl cation symbolize the overlap of
|
2p orbitals of the benzylic C & benzene ring to form bonding MOs
|
|
|
e deficiency & resulting pos charge on benzylic carbocation are shared
|
not only by benzylic C but also by alternate C of the ring
|
|
|
resonance structures of the benzyl cation
|
account for distribution of pos charge calculated from MO theory
|
|
|
resonance stabilizations of the benzyl & allyl cations
|
about the same
|
|
|
structures & stabilities of allylic & benzylic carbocations have important consequences for rxns in which
|
they are involved as reactive intermediates
|
|
|
Rxns in which benzylic or allylic carbocations are formed as intermediates are generally
|
considerably faster than analogous rxns involving comparably substituted nonallylic or nonbenzylic carbocations
|
|
|
the greater reactivities of allylic & benzylic halides result from
|
the stabilities of the carbocation intermediates that are formed when they react
|
|
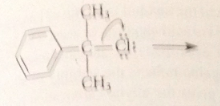
|
|
|
|
|
|
|
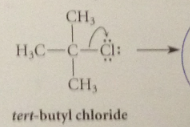
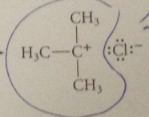
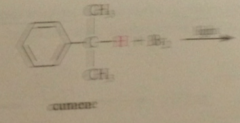
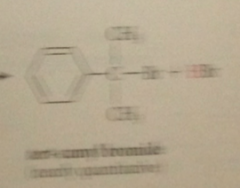
benzylic cation more stable relative to its alkyl halide sm than is
|
tert-butyl cation
|
|
|
bc of possibility of resonance
|
o & p sub groups on benzene that activate EAS further accelerate Sn1 rxns @ benzylic position
|
|
|
carbocation derived from ionization of p-methoxy derivative not only has same types of resonance structures as unsub cmpd
|
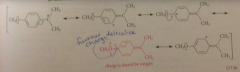
but also an additional structure in which charge can be delocalized onto sub group itself
|
|
|
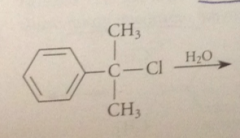
most alcohols require
|
forcing conditions or Lewis acid catalysts to react w HCl to give alkyl chlorides, but such conditions are unnecessary when benzylic alcohols react w HCl
|
|
|
Addition of hydrogen halides to conj dienes
|
also reflects stability of allylic carbocations
|
|
|
protonation of a conj diene gives
|
the allylic carbocation rather than its nonallylic isomer bc the allylic carbocation is formed more rapidly
|
|
|
consequence of involvement of allylic carbocations as reactive intermediates is that
|
in many cases more than one prod can be formed
|
|
|
more than one prod is possible bc the pos charge (& e deficiency)
|
is shared btwn two C
|
|
|
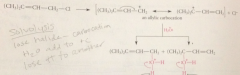
Nuc can react at either of the e deficient C atoms & if 2 C are not equiv
|
2 diff prod result
|
|
|
|
|
|
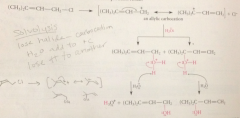
2 prod are derived from one allylic carbocation that has
|
2 resonance forms
|
|
|
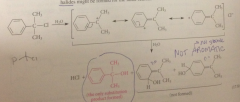
|
|
|
prod derived from rxn of water @ ring C
|
are not formed
|
|
|
Products are not aromatic and thus
|
lack stability associated w the aromatic ring
|
|
|
Allylic radical
|
has an unpaired e at an allylic position
|
|
|
Allylic radicals are resonance stabilized &
|
more stable than comparably substituted nonallylic radicals
|
|
|
Allyl radicalc
|

|
|
|
Benzylic radical
|
unpaired e @ a benzylic position
|
|
|
benzyl radical
|
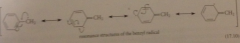
|
|
|
|
|
|
bc allylic & benzylic radical are esp stable
|
they are more readily formed as reactive intermediates
|
|
|
Initiation step
|
dissociation of mlclr bromine into bromine atoms - promoted by heat or light
|
|
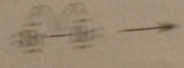
|

|
|
|
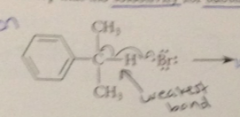
in the first propagation step
|
a bromine atom abstracts the one benzylic H in preference to either the 6 nonbenzylic H or the 5 H of the aromatic ring
|
|
|
It is in this propagation step that
|
the selectivity for sub of the benzylic H occurs
|
|
|
|
|
|
reason for selectivity
|
weaker benzylic C-H bond (greater stability of benzylic radical that is formed)
|
|
|
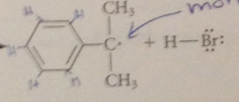
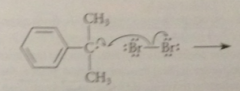
second propagation step
|
benzylic radical reacts w another mlc of bromine to generate a mlc of prod as well as another bromine atom, which can react again
|
|
|
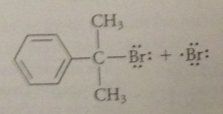
|
|
|
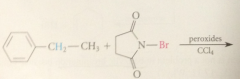
free rad halogenation is used
|
to halogenate alkanes industrially
|
|
|
bc free-rad halogenation of alkanes w diff types of H give smixtures of prod
|
rxn not useful in the lab
|
|
|
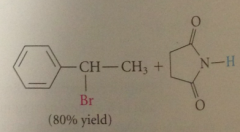
When benzylic H is present
|
undergoes sub so much more rapidly than ordinary H that a single prod obtained
|
|
|
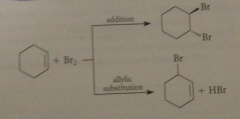
Bc the allylic radical is also relatively stable
|
a similar sub occurs preferentially @ the allylic position of an alkene but a competing rxn occurs in the case of an alkene that is not observed w benzylic sub (addition of halogen to the alkene db by an ionic mech)
|
|
|
|
|
|
one rxn can be promoted over the other
|
if the rxn conditions are chosen carefully
|
|
|
Addition of bromine predominant rxn if
|
free-radical sub is suppressed by avoiding conditions that promote free-rad rxns (heat, light or free-rad initiators) & rxn carried out in solvents of slightly polarity tha tpromote ionic mech for bromine addition
|
|
|
Free-rad sub occurs
|
when rxn promoted by heat, light or free-rad initiators, an apolar solvent such as CCl4 is used & bromine is added slowly so that its conc remains very low
|
|
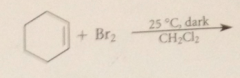
|
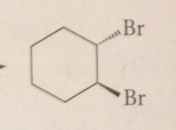
|
|
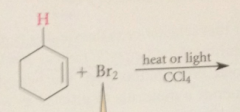
|
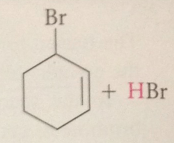
|
|
|
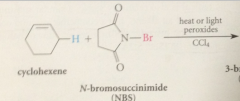
useful reagent employed to accomplish experimental convenience
|
N-bromosuccinimide in CCl4 under free-rad conditions (heat or light & peroxides), allylic bromination takes place & addition to the db is not observed
|
|
|
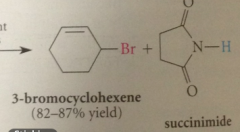
|
|
|
|
|
|
initiation step in allylic and benzylic bromination w NBS
|
is formation of a bromine atom by homolytic cleavage of the N-Br bond in NBS itself
|
|
|
The ensuing substitution rxn has
|
3 propagation steps
|
|
|
First
|
bromine atom abstracts an allylic H from the alkene mlc
|
|
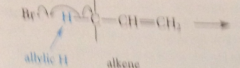
|
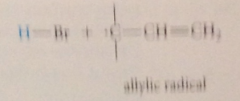
|
|
|
HBr thus formed reacts w
|
the NBS in the second propagation step by an ionic mech to produce a Br2 mlc
|
|
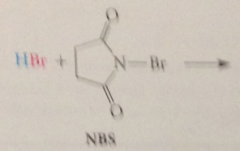
|
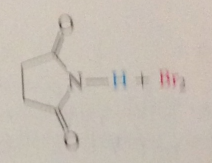
|
|
|
the last propagation step is
|
the rxn of this bromine mlc w the radical formed
|
|
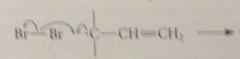
|

|
|
|
the unique role of NBS is
|
to maintain the very low conc of bromine by reacting w HBr
|
|
|
The Br2 conc remains low bc
|
it can be generated no faster than the HBr mlc & an allylic radical are generated
|
|
|
Every time a bromine mlc is formed
|
an allylic radical is also formed w which a bromine can react
|
|
|
the low solubility of NBS in CCl4 is crucial to
|
the success of allylic bromination w NBS
|
|
|
When solvents that dissolve NBS are used
|
diff rxns are observed
|
|
|
CCl4 must be
|
used as the solvent in allylic or benzylic bromination w NBS
|
|
|
During the rxn, the insoluble NBS, which is more dense than CCl4
|
disappears from the bottom of the flask & less dense by-prod succinimide forms a layer on the surface of the CCl4
|
|
|
Many steps of the mechanism
|
occur @ the surface of the insoluble NBS
|
|
|
mix of prod are formed in some allylic bromination rxns bc
|
as resonance structures indicate the unpaired e in the free-rad intermediate is shared by 2 diff C
|
|
|
The prototype for allylic anions is
|
the allyl anion
|
|
|
allyl anion
|

|
|
|
benzyl anion
|
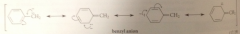
|
|
|
allylic & benzylic anions are more stable than
|
their nonallylic & nonbenzylic counterparts
|
|
|
Reasons for stabilities of anions
|
resonance stabilization, & polar effect of db (in the allyl anion) or phenyl ring (in benzyl anion)
|
|
|
The polar effect of both groups
|
stabilizes anions
|
|
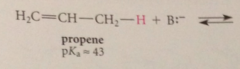
|

|
|
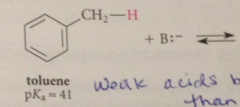
|
|
|
|
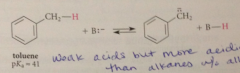
altho these cmpds are very weak acids
|
their acidities are much greater than the acidities of alkanes that do not contain allylic or benzylic H
|
|
|
Free benzylic or allylic carbanions
|
are rarely involved as reactive intermediates
|
|
|
A number of rxns involve species that have
|
carbanion character
|
|
|
Two of these are the
|
rxns of Grignard & related organometallic reagents & E2 elim
|
|
|
Grignard reagents resemble
|
allylic carbanions
|
|
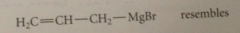
|
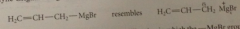
|
|
|
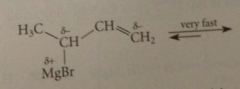
Allylic Grignard reagents undergo
|
a rapid equilibration in which the -MgBr group moves back & forth btwn the 2 partially neg C
|
|
|
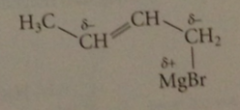
|
|
|
the TS for this rxn can be envisioned as
|
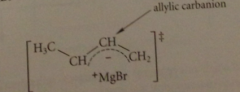
an ion pair consisting of an allylic carbanion and a +MgBr cation
|
|
|
Bc the allylic carbanion is resonance stabilized
|
this TS has relatively low E & equil occurs rapidly
|
|
|
allylic rearrangement
|
simultaneous movement of a group G & db so that one allylic isomer converted into another - not resonance structures, two distinct species in equil
|
|
|
rapid allylic rearrangement of an unsymm Grignard reagent means
|

the reagent is actually a mix of 2 diff reagents
|
|
|
same mix of reagents obtained from
|
either of two allylically related alkyl halides
|
|

|
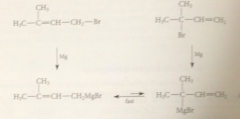
|
|
|
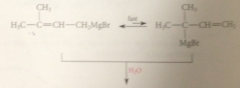
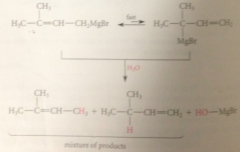
when grignard reagents undergo subsequent rxn
|
mix of prod obtained & same mix obtained regardless of alkyl halide used to form grignard reagent
|
|
|
|
|
|
SN2 & E2 rxns of alkyl halides are
|
competing rxns
|
|
|
major factors that determine which rxn is dominant
|
structure of alkyl halide
|
|
|
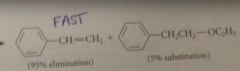
Structural effect in alkyl halide that tends to promote greater fraction of elimination
|
enhanced acidity of B-hydrogens
|
|
|
Greater ratio of elim to sub observed when
|
B-hydrogens of alkyl halide have higher than normal acidity
|
|
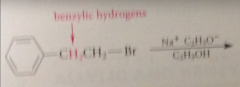
|
|
|
|
Why does acidic B-hydrogen inc rate of E2 rxn?
|
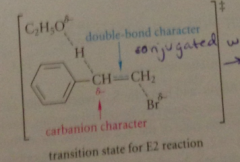
in TS of E2 rxn, base removes B-proton & TS of rxn has carbanion character @ B-carbon atom
|
|
|
partially formed carbanion stabilized in same way that fully formed carbanion is
|
more stable TS results in faster rxn
|
|
|
Another reason benzylic E2 rxns faster
|
alkene db, partially formed in TS, conj w benzene ring
|
|
|
SN2 rxns of allylic & benzylic halides are
|
relatively fast
|
|
|
allylic & benzylic SN2 rxns are accelerated bc
|
the energies of their TS are reduced by 2p orbital overlap
|
|
|
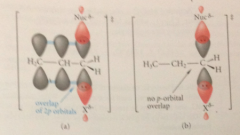
In the TS of the SN2 rxn
|
the C at which sub occurs is sp2 hybridized: incoping nuc & departing LG partially bonded to a 2p orbital on this C
|
|
|
Overlap of 2p orbital w 2p orbitals of adjacent db or phenyl ring provides
|
additional bonding that lowers the E of the TS & accelerates the rxn
|
|
|
orbital overlap
|
|
|
|
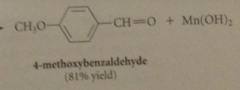
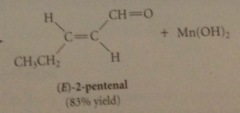
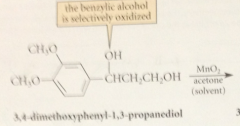
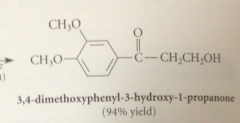
allylic & benzylic alcohols are oxidized selectively by
|
suspension of activated manganese (IV) dioxide, MnO2
|
|
|
primary allylic alcohols oxidized to
|
aldehydes
|
|
|
secondary allylic alcohols oxidized to
|
ketones
|
|
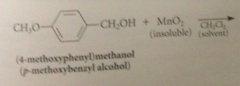
|
|
|
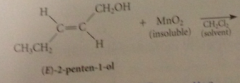
|
|
|
|
Acitvated MnO2 is obtained by
|
oxidation-reduction rxn of potassium permanganate, KMnO4, w Mn2+ salt such as MnSO4 under either alkaline or acidic conditions followed by thorough drying
|
|
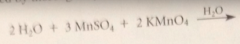
|
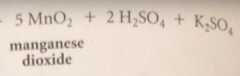
|
|
|
Allylic & benzylic oxidation of alcohols takes place on
|
surface of the MnO2, which is insoluble in solvents used for the rxn
|
|
|
Water competes w alcohol for sites on MnO2 and thus
|
must be removed by drying to produce an active oxidant
|
|
|
|
|
|
Rxn is selective bc
|
allylic & benzylic alcohols react much more rapidly than ordinary alcohols
|
|
|
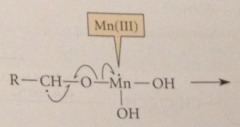
In first step of mech
|
OH group of alcohol rapidly adds to MnO2 to give ester
|
|
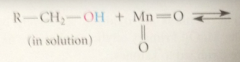
|
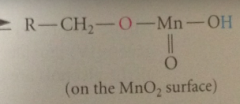
|
|
|
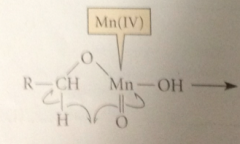
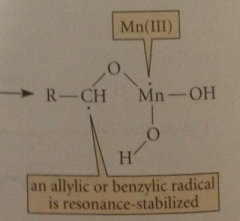
Next step (RLS)
|
Mn(IV) accepts an e to become Mn(III) & H atom transferred from allylic or benzylic C to an O of the oxidant
|
|
|
prod has an unpaired e on the
|
allylic or benzylic C & is therefore resonance stabilized radical
|
|
|
|
|
|
Allylic/benzylic selectively occurs bc
|
analogous radical intermediate in oxidation of an alcohol that is not allylic or benzylic is less stable & formed more slowly
|
|
|
In final step rapid, Mn(III) is reduced to more stable Mn(II)
|
& strong C=O db formed to give aldehyde prod, which is washed away from oxidant surface by solvent
|
|
|
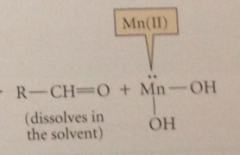
|
|
|
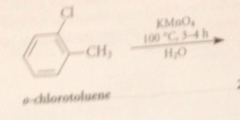
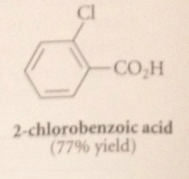
treatment of alkylbenzene derivatives w strong oxidizing agents under vigorous conditions converts
|
alkyl side chain int oa carboxylic acid group
|
|
|
Oxidants commonly used for this purpose
|
Cr(VI) derivatives i.e. Na2Cr2O7 (sodium dichromate) or CrO3, K2MnO4 (potassium permanganate) or O2 & special catalysts
|
|
|
|
|
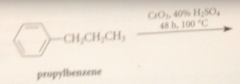
|
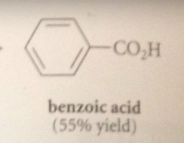
|
|
|
alkyl side chain, regardless of length
|
converted into a CA group
|
|
|
Oxidation of alkyl side chains requires
|
presence of a benzylic H
|
|
|
tert-butylbenzene, which has no benzylic H
|
resistant to benzylic oxidation
|
|
|
Benzylic oxidations occur in many cases bc
|
resonance stabilized benzylic intermediates i.e. benzylic radicals are involved
|
|
|
conditions for side-chain oxidation
|
generally vigorous: heat, high conc oxidant and/or long rxn times
|
|
|
1-phenylethanol readily oxidized to acetophenone under
|
milder conditions - normal oxidation of secondary alcohols to ketones - but converted into benzoic acid under more vigorous conditions
|
|
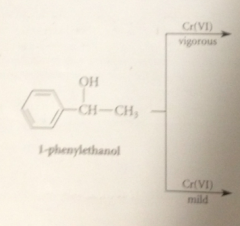
|
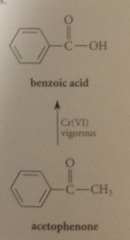
|
|
|
essential oil
|
possessed key characteristic i.e. odor or flavor of natural material from which it comes
|
|
|
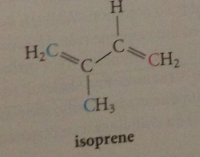
isoprene rule
|
terpenes all consist of repeating units w same C skeleton as 5-C diene isoprene
|
|
|
isoprene
|
|
|
|
basis of terpene or isoprenoid classification
|
connectivity of C skeleton
|
|
|
C-4 is
|
either C of dimethyl branch
|
|
|
many terpenes: isoprene units connected in
|
1-4 arrangement, so C-4 of one skeleton connected to C-1 of other
|
|
|
prime on one number, absence on other mean
|
connection is between diff isoprene units
|
|
|
some cmpds derived from conventional terpene structures by
|
skeletal rearrangements
|
|
|
criteria by which to recognize terpenes
|
multiple of 5 C atoms in main C skeleton
|
|
|
main connectivity C of isoprene C skeleton
|
within each 5 carbon unit
|
|
|
terpene C skeletons contain
|
multiples of 5 C atoms
|
|
|
monoterpenes
|
terpenes w 10 C atoms in carbon chains
|
|
|
sesquiterpenes
|
15 C
|
|
|
diterpenes
|
20 C
|
|
|
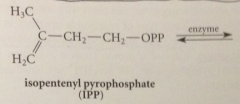
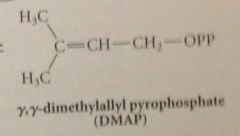
repetitive isoprene skeleton in all terpenes has a common origin in
|
two simple five C cmpds
|
|
|
|
|
|
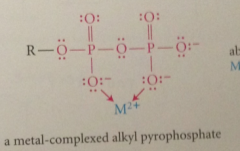
pyrophosphate group
|
|
|
|
alkyl pyrophosphates are esters of
|
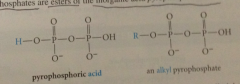
inorganic acid pyrophosphoric acid
|
|
|
pyrophosphate & phosphate are
|
nature's LG
|
|
|
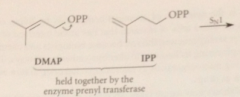
biosynth of simple monoterpene geraniol: first step
|
IPP & DMAP bound to enzyme prenyl transferase
|
|
|
DMAP loess pyrophosphate LG in
|
SN1-like process
|
|
|
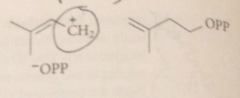
|
|
|
carbocation formed is
|
relatively stable allylic cation
|
|
|
carbocations like other electrophiles
|
can react w pi e of a db, which acts as a nuc
|
|
|
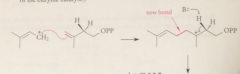
Rxn of carbocation w db of IPP gives
|
new carbocation
|
|
|
Loss of a proton from a B-C of this carbocation gives
|
the monoterpene geranyl pyrophosphate
|
|
|
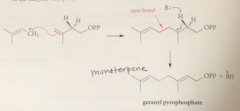
|
|
|
geraniol is formed in the rxn of
|
water w geranyl pyrophosphate
|
|
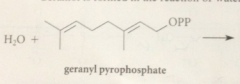
|
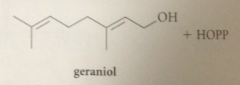
|

