![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
35 Cards in this Set
- Front
- Back
|
Define: Element |
a pure substance that cannot be broken down into a simpler substance - SMALLEST UNIT OF MATTER |
|
|
Define: COMPOUND |
a pure substance that is made of more than atom or element |
|
|
Define: ATOM |
smallest unit of matter |
|
|
Define: MOLECULE |
two or more elements combined; can be an element (H2, N2, O2) or a compound (CO2, H20) |
|
|
True or False: a MOLECULE can be EITHER an ELEMENT or a COMPOUND |
true |
|
|
Define: PURE SUBSTANCE |
made of ONLY ONE THING. i.e - Pure Element, Pure Molecule, Pure Compound |
|
|
Define: MASS |
the amount of matter in an object |
|
|
Define: VOLUME |
the amount of SPACE an object takes |
|
|
Define: DENSITY |
the amount of matter in a GIVEN SPACE |
|
|
Define: INDEPENDENT VARIABLE |
the part of the experiment that you MEASURE |
|
|
Define: INDEPENDENT VARIABLE |
the ONE thing in an experiment that is CHANGED or MANIPULATED |
|
|
What is the formula to calculate DENSITY? |
Density = Mass / Volume (D = M/V) |
|
|
If Density = Mass divided by Volume (D=M/V), then how do you calculate volume? |
Volume (V) = Mass (M) / Density (D) |
|
|
True or False: ATOMS are the SMALLEST PARTICLE of a substance? |
True |
|
|
How many elements are found on the periodic table? |
? |
|
|
True or False:Elements are smaller than Atoms? |
False! Atoms are the smallest particle of a substance |
|
|
How many naturally occurring elements are there? |
92 |
|
|
The current version of the periodic table is by: Mendeleev or Mendelsohn? |
Mendeleev |
|
|
What year was the current version of the period table created? |
1869 |
|
|
True or False:Elements do not have unique physical and chemical properties |
False! Elements DO have unique physical and chemical properties |
|
|
Can elements be made in the laboratory? |
yes |
|
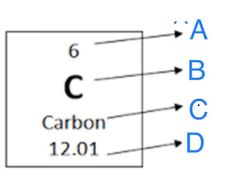
Does "A" represent the Atomic Number or the Atomic Mass? |
Atomic Number |
|
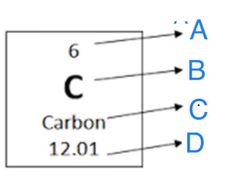
What does "B" represent in the image? |
Element Symbol |
|
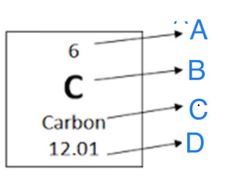
True or False: Atomic Mass is represented by "D" in this image. |
True |
|
|
What is the chemical symbol for Oxygen? |
O |
|
|
True or False: a chemical symbol can only be one letter |
False. A chemical symbol can be one letter or two. |
|
|
True or False: if the chemical symbol has two letters, the FIRST LETTER IS ALWAYS CAPITALIZED and the second letter is always lowercase |
True! |
|
|
True or False: Atoms can be the same element or different element |
True |
|
|
True or False: TWO OR MORE atoms combined are a MOLECULE |
True |
|
|
What is the difference between an ELEMENT molecule and a COMPOUND molecule? |
An ELEMENT Molecule is made up of 2 or more of the same element (O2) A COMPOUND Molecule is made of of 2 or more of TWO OR MORE DIFFERENT elements (H2O) |
|
|
PRO-TIP! One capital letter = element Two or more capital letters = compound |
Boom! |
|
|
Is H2SO4 an ELEMENT or a COMPOUND? |
Compound! |
|
|
Is "Fe" an element or a compound? |
element! |
|
|
"CH4 + O2" -- Identify how many compounds, elements, and atoms of each element are in the equation |
Compounds: 1 (CH4) Elements: 3 (C, H, and O) Atoms of each Element: 1 atom of C 4 atoms of H 2 atoms of O |
|
|
True or False: Some element names come from Latin or Greek |
True |

