![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
252 Cards in this Set
- Front
- Back
|
What is the difference between epidural and subdural bleeding?
|
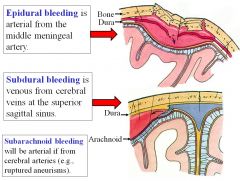
Epidural bleeding is arterial from the middle meningeal artery (high pressure system), while subdural bleeding is venous from cerebral veins at the superior sagittal sinus (lower pressure system).
|
|
|
What type of head injury is often characterized as “the worst headache of my life?”
|
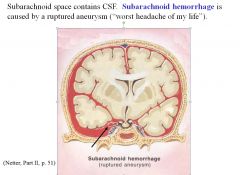
Subarachnoid hemorrhage
|
|
|
What cells are the source of meningiomas?
|
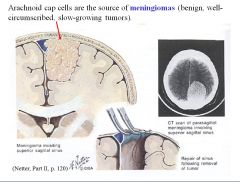
Arachnoid cap cells
|
|
|
At what level is a lumbar puncture performed?
|
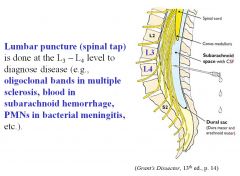
L3-L4
|
|
|
What disorder is usually seen in the elderly and characterized by dementia, ataxia, and urinary incontinence?
|
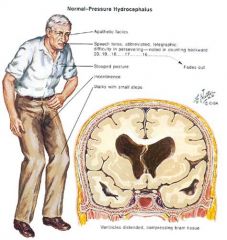
• Normal pressure/nonobstructive communicating hydrocephalus.
• (remember: wacky, wobbly, and wet). • It’s typically caused by scarring of the pia-arachnoid and arachnoid villi preventing resorption of the CSF into the superior sagittal sinus. |
|
|
What type of stroke causes monocular blindness?
|
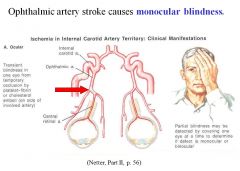
• Ophthalmic artery stroke causes monocular blindness; the lesion is anterior to the optic chiasm
• Any lesion posterior to the optic chiasm will affect both eyes |
|
|
What would be the effects of a stroke in the middle cerebral artery? What about a stroke in the anterior cerebral artery?
|
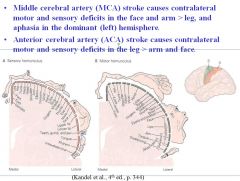
• Middle cerebral artery (MCA) stroke causes contralateral motor and sensory deficits in the (face and arm) > leg, and aphasia in the dominant (left) hemisphere.
• Anterior cerebral artery (ACA) stroke causes contralateral motor and sensory deficits in the leg > (arm and face). |
|
|
What type of lesion would cause hemiplegia where the face=arm=leg?
|
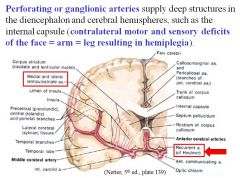
Stroke/occlusion of a perforating or ganglionic artery, leading to a lesion of the internal capsule.
|
|
|
What would be the effects of a stroke in the posterior cerebral artery?
|
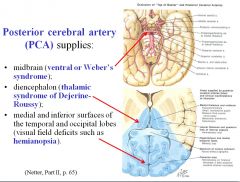
A stroke of the branch supplying the midbrain would produce ventral or Weber’s syndrome (CN 3 palsy and contalateral hemiplasia).
A stroke of the branch supplying the diencephalon would cause thalamic syndrome of Dejerine Roussy. A stroke of the branch supplying medial and inferior surfaces of the temporal and occipital lobes would produce contralateral blindness in visual field of each eye (hemianopsia). |
|
|
What is the most common site of aneurysm in the human body?
|
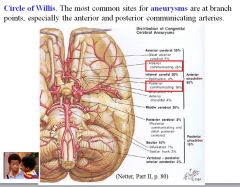
The circle of Willis.
|
|
|
What would be the effects of a stroke in the posterior inferior cerebellar artery (PICA)?
|
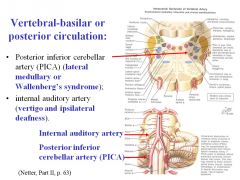
A stroke to PICA would cause Wallenberg’s syndrome, resulting in alternating hemianesthesia:
• Contralateral loss of pain and temperature from the body and ipsilateral loss of pain and temperature from the face • Dysphagia, dysphonia and dyspnea caused by ipsilateral paralysis of the muscles of the larynx, pharynx and soft palate (nucleus ambiguous and IX, X nerves). When patient says, “ah,” the uvula deviates to the opposite (good) side |
|
|
What would be the result of a stroke of the internal auditory artery?
|
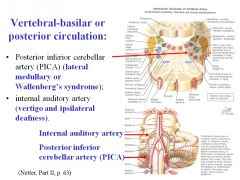
A stroke of the internal auditor artery would cause vertigo and ipsilateral deafness.
(distribution of internal auditory artery is to the inner ear (cochlea and canals); accompanies vestibulocochlear nerve into the inner ear) |
|
|
What sensations are carried in the dorsal column ascending tract?
|
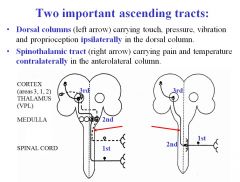
Dorsal column carries touch, pressure, vibration and proprioception ipsilaterally .
|
|
|
What sensations are carried in the spinothalamic ascending tract?
|
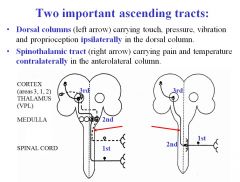
Pain and temperature are carried in the ascending spinothalamic tract contralaterally in the anterolateral column.
|
|
|
What would be the result of compression of a dorsal root?
|
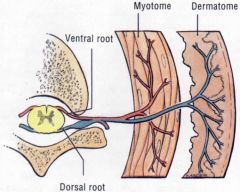
• Ipsilateral paresthesia, pain or anesthesia (numbness) in the affected dermatomes and decreased or absent deep tendon reflexes..
• If the compression occurs slowly, the progression is parathesia, pain, and numbness. If it occurs quickly, you bypass the parathesia stage and go right to pain and numbness. |
|
|
What would be the result of compression of a ventral root?
|
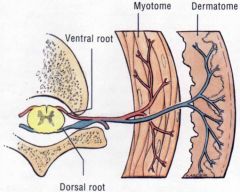
Ipsilateral muscle weakness (paresis) or flaccid (limp) paralysis in the affected myotomes and decreased or absent deep tendon reflexes.
|
|
|
What would be the related symptomatology of complete transection of the spinal cord?
|
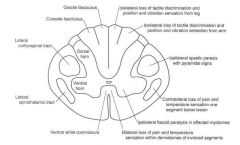
Complete transection of the spinal cord results in bilateral signs and symptoms below the level of the lesion:
• Loss of all sensation (anesthesia) producing a sensory level, • Initial flaccid paralysis (spinal shock) followed by UMN signs (spastic paralysis with hyperreflexia and Babinski sign). |
|
|
What would be the related symptomatology of hemisection of the spinal cord (Brown-Sequard syndrome)?
|
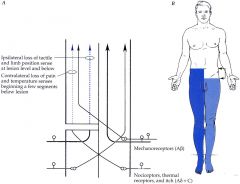
• ipsilateral loss of touch, pressure, vibration and proprioception at and below the lesion level (dorsal column),
• ipsilateral UMN signs (spasticity) at and below the lesion level (lateral corticospinal tract), • contralateral loss of pain and temperature beginning one or two segments below the lesion level (spinothalamic tract). • ipsilateral anesthesia and LMN signs at the level of the lesion. |
|
|
What is one condition we discussed in lecture that can cause bilateral pain and temperature sensation loss over several dermatomes?
|
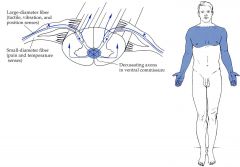
Central cord syndrome or syringomyelia, caused by fluid-filled cavity (syrinx) in the center of the cervical cord that interrupts pain and temperature fibers from both sides crossing in the ventral white commissure resulting in a bilateral band of analgesia and thermanesthesia.
|
|
|
What type of lesion gives you contralateral paralysis of the lower face only?
|
UMN lesion
|
|
|
What type of lesion gives you ipsilateral paralysis of upper AND lower face?
|
LMN lesion
(LMN lesion of facial nerve = Bell’s palsy) |
|
|
What would be the symptomatic result of a lesion (e.g., stroke) in the internal capsule)?
|
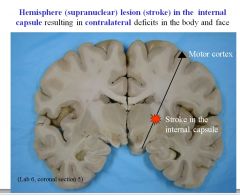
Lesion in the internal capsule results in contralateral deficits in the body and face.
|
|
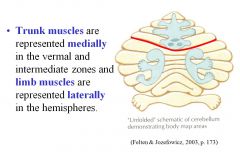
What is the result of midline vermal lesions?
|

Midline vermal resions result in truncal ataxia with a wide based gait:
Positive Romberg sign Falling toward the lesion side Nystagmus (involuntary beating movements of the eyes) |
|
|
Which of the following is NOT a result of a lateral hemisphere lesion (of the cerebellum):
A) limb ataxia B) hyperreflexia C) intention tremor D) limb dysmetria (over or undershoot the target) E) dysarthria (abnormal articulation) |
Answer: hyperreflexia; actually hyporeflexia results from a lateral hemisphere lesion
|
|
|
What would be the result of a stroke in the subthalamic nucleus?
|
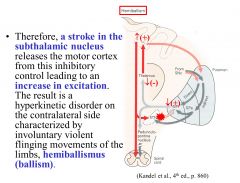
The subthalamic nucleus constitutively inhibits the cortex, thus a stroke in the subthalamic nucleus would cause an increase in excitation, resulting in hyperkinetic disorder on the contralateral side characterized by violent flinging movements of the limbs (hemiballismus).
|
|
|
Can you explain why degeneration of dopaminergic neurons (e.g., in Parkinson’s disease) causes rigidity, dystonia, akinesia, and bradykinesia?
|
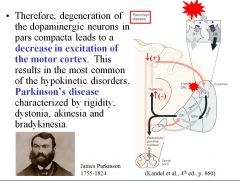
Dopaminergic neurons are responsible for disinhibiting the thalamus, thus facilitating movements initiated in the cortex. Deletion of these pathways thus results in a decrease in excitation of the motor cortex, resulting in rigidity, dystonia, akinesia, and bradykinesia.
|
|
|
What is Thalamic syndrome of Dejerine-Roussy/a lesion of what artery causes it?
|
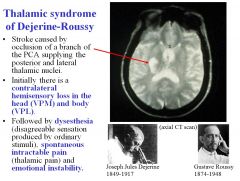
Thalamic syndrome of Dejerine Roussy is caused by a stroke occluding a branch of the PCA supplying the posterior and lateral thalamic nuclei.
Initially there’s contralateral hemisensory loss in the head (VPM) and body (VPL) Afterwards, there’s dysesthesia, spontaneous intractable pain, and emotional instability. |
|
|
What are the most common primary sites causing brain metastatic spread?
|
• Lung
• Breast • Melanoma |
|
|
What is the classic MRI appearance of a glioblastoma multiforme (GBM)?
|
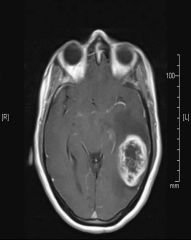
Enhancing lesion with central necrosis
|
|
|
What is the treatment/prognosis for GBM?
|
• Surgery (to reduce mass effect, edema, and tumor volumetemozolomide (chemo) +RT adjuvant temodar
• Treatment is not curative • New agents are being investigated in clinical trials • Median survival is about 15 months |
|
|
What grade tumors are more likely to have seizures?
|
Low grade tumors are more likely to have seizures.
|
|
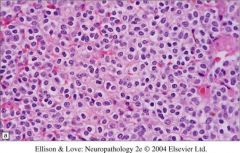
What type of tumors have fried egg appearance?
|
Oligodendrogliomas have fried egg appearance; they also have chicken-wire capillaries.
|
|
|
What’s the most common presentation for oligodendrogliomas?
|
Focal seizures in a young adult. Because they grow more slowly than astrocytomas, there’s often a prolonged period of symptoms (usually focal seizures) prior to diagnosis.
|
|
|
What chromosomal marker would predict a good prognosis for oligodendrogliomas?
|
1p and 19q loss of heterozygosity
|
|
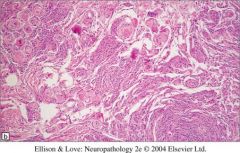
What type of tumor is this?
|
It’s a psammommatous meningioma
• Intracranial tumors that are thought to arise from the meningothelial arachnoid cap cells of the meninges • Overwhelming majority are benign (Grade I), slow growing tumors that compress the underlying brain, but rarely invade it • Median age at diagnosis is 64 years • Genetic predisposition (neurofribromatosis type 2) and prior exposure to ionizing radiation are the only known risk factors • Focal seizures and neurologic deficits from brain or cranial nerve compression are the most common presenting symptoms |
|
|
What unilateral, slow-growing tumor is pathognomonic for NF2?
|
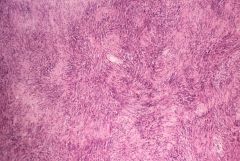
Schwannoma
• Usually present with gradual unilateral hearing loss, sometimes preceded by tinnitus and a vague feeling of “dizziness.” |
|
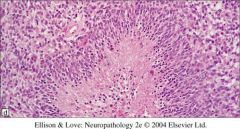
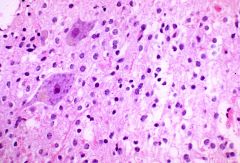
What’s the description of this type of feature?
|
Pseudo palisading (characteristic of glioblastoma)
It’s an aggressive, rapidly dividing tumor, so there’s hypercellularity |
|
|
What’s the most common presenting symptom for CNS lymphoma?
|
Behavioral change and cognitive impairment
|
|
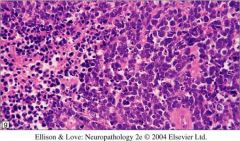
If you see the following words: hypercellular, small round blue cells, sheet-like growth, what will you think?
|
Medulloblastoma (or some other germ cell tumor)
• Malignant tumor of childhood located in the vermis of the cerebellum • Tendency to seed the leptomeninges and cause widespread metastases within the CNS and occasionally extra-neural metastases • Most common intracranial tumor of children (25% of childhood brain tumors) • Median age of onset is 7 years • Pathology = “small blue cell tumor” |
|
|
What is GFAP, and what is it used for?
|
GFAP stands for glial fibrillary acidic protein; it is a cytoplasmic fibrillary protein characteristic of astrocytes and can be used as a stain to identify tumors of astrocytic origin.
|
|
|
What brain tumor is commonly located in the spinal cauda equina?
|
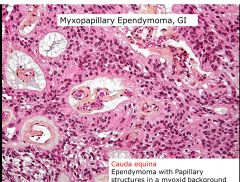
Myxopapillary Ependymoma
|
|
|
What histopathologic criteria are there for diffuse astrocytoma, and what grade is it?
|
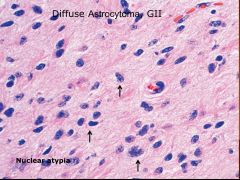
Just one criteria: nuclear atypia.
Grade 2 |
|
|
What histopathologic criteria are there for anaplastic astrocytoma, and what grade is it?
|
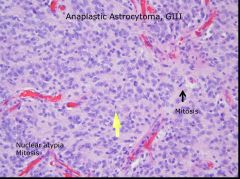
2 criteria: nuclear atypia and mitosis
Grade 3 |
|
|
What histopathologic criteria are there for glioblastoma, and what grade is it?
|
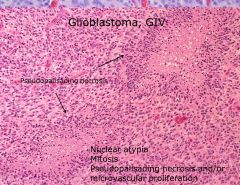
• Nuclear atypia
• Mitosis • Pseudopalisading necrosis • Microvascular proliferation |
|
|
Which brain tumor is associated with NF1?
|
Pilocytic astrocytoma
|
|
|
What brain tumor is associated with NF2?
|
Ependymoma
|
|
|
What is a common location for Ependymoma?
|
The 4th ventricle; common in children
Spinal in adults Generally arises around ventricle lining |
|
|
Which brain tumor has “chicken wire capillaries?”
|
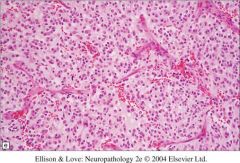
Oligodendroglioma
|
|
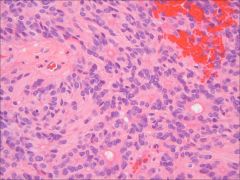
Which brain tumor is associated with “rosettes?”
|
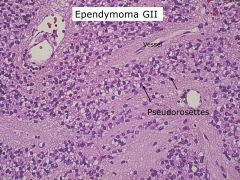
Ependymoma; also associated with pseudorosettes
|
|
|
What tumor(s) is/are associated with small blue cells?
|
Embryonal tumors:
• Medulloblastoma (20% of pediatric brain tumors) • Primitie Neuroectodermal Tumor of the CNS (PNET) • Atypical Teratoid/Rhabdoid Tumor (ATRT) |
|
|
What are the two components of consciousness?
|
• Arousal
• Content |
|
|
What are the 3 main anatomical components of consciousness?
|
1. Ascending arousal system (reticular activating system)
2. Thalamus and hypothalamus 3. Cerebral cortex |
|
|
Can you describe the Glasgow Coma Scale (GCS)?
|
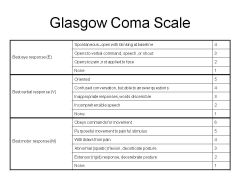
(see image)
|
|
|
What are some causes of Locked-In Syndrome?
|
Locked in syndrome is when there is no motor output except vertical movement of the eyes. In its purest form, the following are characteristics:
• Paralysis of all four limbs and the lower cranial nerves • Most commonly caused by lesions in the base/tegmentum of pons • Typically retain vertical eye movements It can be caused by pontine lesions or severe neuromuscular disease. |
|
|
What is akinetic mutism?
|
• Akinetic mutism is a disorder of motivation characterized by a lack of volitional activity. Classically, patients may even appear hypervigilant. External evidence for mental activity remains almost entirely absent and spontaneous motor activity is lacking.
• Classically due to medial frontal lobe lesions and lesions of the hypothalamus and adjacent basal forebrain. • Note that one of the key differences between this and vegetative state is the presence of visual tracking, which may be the only way to distinguish these cases from the vegetative state. • Exam shows no evidence of damage to motor pathways. |
|
|
What are the exam criteria for brain death?
|
1. Unresponsive
2. Absence of brainstem reflexes: a. No pupillary response b. No eye movement c. No corneal reflex d. No grimace to pain e. No gag/cough 3. Apnea Need to have normal core temperature and normal systolic blood pressure. Brain death = death |
|
|
What drug antagonizes glutamate’s effects on the brain (ischemia and death)?
|
Citicoline.
Glutamate has the following negative effects: • Glutamate is a toxic excitatory neurotransmitter • Glutamate produces a characteristic pattern of neuronal death o dendrites and soma destroyed • axons, presynaptic terminals, non-neuronal cell survival • Reverses the sodium-calcium pump o Exacerbates calcium buildup |
|
|
Can you use hypothermia to treat traumatic brain injury (TBI) patients?
|
No; hypothermia does NOT improve outcome in TBI
• Hypothermia is the only neuroprotectant in clinical use • Reduces neuronal energy demand o Decreased oxygen, ATP, glucose consumption • Proved efficacy after cardiac arrest with return of circulation |
|
|
Are there any measures mentioned during the traumatic brain injury lecture that can ameliorate brain herniation?
|
23.4% Saline Reduces ICP and improves GCS in the setting of herniation
|
|
|
Can you give steroids to treat a patient with traumatic brain injury (TBI)?
|
The only class I recommendation for TBI is: don’t give steroids. It makes patients more likely to die
|
|
|
What resuscitation fluid should be given for traumatic brain injury (TBI) patients?
|
Pts should get crystalloid for volume resuscitation
(mechanism unclear) |
|
|
Which type of hematoma is under arterial pressure? Which one is under venous pressure?
|
• Epidural hematoma – under arterial pressure
• Subdural hematoma – under venous pressure |
|
|
How does one increase brain oxygen tension, and why would you want to (in the context of traumatic brain injury)?
|
Brain oxygen tension can be increased by increasing the FIO2, the cerebral perfusion, or packed RBC transfusion.
Patients with higher brain O2 tension tend to have better outcomes than patients with lower brain O2 tension. |
|
|
What are hallmark symptoms associated with Parkinson’s disease? (Think TRAP)
|
• Tremor resting
• Rigidity • Akinesia • Postural instability |
|
|
What are important risk factors for Parkinson’s disease?
|
• Age is strongest predictor of increased risk
• Other risk factors include: – Exposure to environmental toxins • Herbicides, pesticides, heavy metals – Genetic factors; family history of parkinsonism • <5% have genetic cause • Multiple genes identified – Male gender |
|
|
What are the pathological hallmarks of Parkinson’s disease?
|
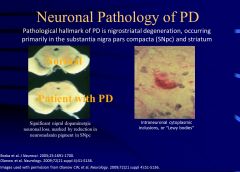
Nigrostriatal degeneration is a pathological hallmark of PD, occurring primarily in the substantia nigra pars compacta (SNpc) and striatum)
Also, Lewy bodies are a histopathological hallmark of PD |
|
|
What disease is characterized by Parkinsonism, dysarthria + dysphagia, and an impairment of vertical eye movement?
|
Progressive Supranuclear Palsy
|
|
|
How might you distinguish between the fairly similar Multiple System Atrophy and Parkinson’s disease?
|
PD pts never lose total response to levodopa, but in MSA, they can lose response to the drug
|
|
|
How would you distinguish between resting tremor that’s characteristic of Parkinson’s disease (PD) and that of Essential tremor? Furthermore, what non-neurological organ should be examined if a patient presents with tremor?
|
• PD – head doesn’t shake. ET - head can shake.
• Hyperthyroidism is a common cause of tremor, so the first thing they do when they see someone with tremor is they check their thyroid. Essential tremor is activity triggered; ET won't have any other symptoms. |
|
|
What should be on your differential for chorea?
|
• Huntington disease (genetic testing)
• Drugs (levodopa, phenytoin, amphetamines,oral contraceptives, lithium • CNS infections • CNS toxins • Neuroacanthocytosis (blood smear) • Wilson’s disease (ceruloplasmin, urine Co, KF) • Lupus (ANA) • Senile chorea (Dx of exclusion) |
|
|
What is dystonia?
|
• Sustained but not fixed muscle contraction that can cause twisting or repetitive movements and abnormal postures
– Agonists and antagonist muscle co-contract – Initially brought out by action and not present at rest |
|
|
What is myoclonus?
|
• Brief, “lightning”-like muscle jerks
|
|
|
Why would you not want to give anticholinergics to an elderly patient with Parkinson’s disease?
|
Anticholinergics only treat tremor and don’t really affect the other symptoms (e.g., bradykinesia and rigidity). More importantly, the side effect profile is very limiting: nausea, blurred vision, urinary retention, tachycardia, *confusion*. Thus, you would really only consider anticholinergics in young patients with tremor dominant disease and minimal other parkinsonian signs/symptoms.
|
|
|
What is an important side effect of dopamine agonists (e.g., praxipexole, ropinirole) that may require counseling?
|
Impulse Control Disorders (ICDs):
• Compulsive buying • Compuslve gambling • Binge eating • hypersexuality |
|
|
What is the gold standard treatment for Parkinson’s disease, and what are important side effects?
|
Levodopa
Side effects: • Dyskinesias • Motor fluctuations |
|
|
Match the type of patient with the starting therapy: older patient, younger patient, levodopa, dopamine agonist.
|
Younger patients are more prone to developing levodopa induced motor complications, so initial treatment with an agonist is generally recommended in this population
Early use of levodopa is recommended in the older population because they are more sensitive to neuropsychiatric adverse reactions of the agonists and they are less prone to developing |
|
|
What treatment(s) would you use for Essential Tremor patients?
|
• Beta-blockers
• Primidone (an anticonvulsant) |
|
|
What treatment(s) would you use for chorea?
|
• Neuroleptics
• Dopamine-depleting agents – Tetrabenazine • Benzodiazepines – Clonazepam |
|
|
What are weak opioids? What are strong opioids?
|
• Weak
– Codeine – Maybe hydrocodone (NOT oxycodone) • Strong – Everything else – E.g. morphine, heroin, hydromorphone, etc. • Excluded: antitussives and antidiarrheals |
|
|
What are the 2 opioid mu antagonists?
|
• Naloxone
• Naltrexone |
|
|
What is the only opioid partial agonist?
|
• Buprenorphine
• Binds mu with HIGH affinity (higher than most opioids), which makes naloxone reversal difficult in OD • Current use: opioid maintenance |
|
|
What are the opioid agonist/antagonists (Kappa agonists but Mu antagonists)?
|
• Pentazocine (don’t really need to know this one)
• Butorphanol • Nalbuphine |
|
|
What genetic process is responsible for opioid receptor subtypes?
|
One gene codes for each opioid receptor: Mu, Kappa, Delta
Subtypes come from splice variants |
|
|
What organ system has the greatest number of opioid receptors outside the CNS?
|
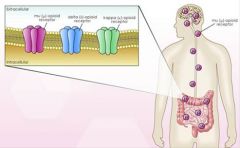
The GI tract has the greatest number of opioid receptors outside the CNS.
|
|
|
What do you do if when titrating an opioid up, the patient gets drowsy and nauseous without any reduction in the pain?
|
Change the opioid!
|
|
|
What types of fibers do opioids generally work on?
|
Opioids work principally work on C fibers; they don’t work on touch fibers or movement fibers
|
|
|
What are the elimination half-lives for the following drugs: 1) morphine, meperidine, hydromorphone, oxycodone, 2) methadone 3) fentanyl
|
1) morphine, meperidine, hydromorphone, oxycodone – 2-3 hr
2) methadone – notable at 24 hr 3) fentanyl – 3 hr |
|
|
What are the major effects of opioids?
|
• Analgesia
– Both sensory and emotional parts • Respiratory Depression • Sedation or “euphoria” – Sometimes dysphoria – NOT AMNESIA!! • Miosis (need to know this) – Excessive constriction of the pupil – No tolerance to this (thus useful for overdose dx) – Mediated by parasympathetic, so can be blocked by atropine – Seen with all opioid agonists • Slowed peristalsis – Via enteric NS opioid receptors • Nausea/vomiting – Via chemoreceptor trigger zone • Increased sphincter tone – Bladder, sphincter of Oddi |
|
|
What are major effects of opioids that you do not develop tolerance to?
|
• Mioisis
• Constipation |
|
|
How would you treat the following opioid side effects: nausea, constipation, itching, sedation?
|
• Nausea: phenothiazines, droperidol
• Constipation: the usual • Itching: antihistamines (diphenhydramine) • Sedation: amphetamines (end stage CA ONLY!) • You can use opioid antagonist or agonist/antagonist for any of these, but there’s always a risk you may reverse the analgesia |
|
|
What are clinical signs of opioid overdosage, and how would you treat?
|
• Clinical signs
o Respiratory depression (decreased CO2 response and slow rate) o Unresponsive o mioisis • Treatment o ABCs! – support ventilation o Antagonist – naloxone |
|
|
What are the breakdown products of Morphine?
|
Morphine gets broken down in the liver into:
• Morphine 3-Glucoronide (minimal activity) • Morphine 6-Glucoronide (very potent) Normally, Glucoronide can’t enter CNS. In renal failure, M6G can enter CNS by mass effect. |
|
|
Which opioid can be used in a patient with renal failure?
|
Hydromorphone (dilaudid)
|
|
|
Why is it that 7-10% of the population would not be responsive to codeine?
|
• Codeine as methymorphine has low receptor affinity and must be metabolized to morphine, which 7-10% of the population cannot do.
• Codeine is considered a weak opioid • Not as popular, because it’s not as potent. • Considered less addictive. |
|
|
Why should pill form of oxycontin not be crushed prior to administration?
|
Oxycontin is the sustained release pill form of oxycodone, but in order for the sustained release to work, it must not be crushed. The pill must be intact.
|
|
|
Can meperidine be used in renal failure patients?
|
Meperidine is an opioid.
• No, it cannot be used in renail failure patients, because it’s cleared by the kidneys. • Has 10 hour half-life. • Metabolite = normeperidine • Can cause malignant neuroleptic syndrome |
|
|
Why would an agonist/antagonist opioid be used?
|
• To reverse itching (and other opioid side effects) while still maintaining analgesia. That’s how they’re used; they’re not used a lot.
• Do NOT use in opioid tolerant patient, because it triggers withdrawal. |
|
|
What sensations do A-beta, A-delta fibers and C fibers carry?
|
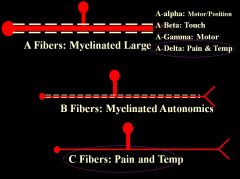
• Need to know A-beta only carries touch, and A-delta only carries pain and temp (quickly).
• C fibers – slow, diffuse, highly aversive pain (biggest problem for pain doctors) |
|
|
What does allodynia mean? What does hyperalgesia mean?
|
Allodynia: pain with touch (e.g., sunburn)
Hyperalgesia: amplified pain |
|
|
What is your goal for pain management (i.e., what number on a scale from 0-10 are you going for)?
|
Your goal is 4 or less, not 0, because a goal of 0 pain could end up with the patient OD’d or the patient may run into other problems.
|
|
|
Which of the following is not an example of multimodal analgesia:
A) Opioid + NSAID B) Opioid + acetaminophen C) Opioid + cold D) Ibuprofen + aleeve |
D; Multimodal analgesia combines drugs with different mechanisms for a synergistic effect with reduced side effects.
|
|
|
How can antidepressants be helpful in analgesia?
|
• Antidepressants block reuptake of both serotonin and NE in the dorsal horn of the spinal cord.
• They enhance the descending inhibitory system. • Need 2-4 weeks to see effect. • Effect is mild-moderate in only 70% |
|
|
What are the available anti-epileptic drugs used for pain that we discussed during lecture?
|
• Carbamazepine (Tegretol) – first used in trigeminal neuralgia
• Gabapentin (Neurontin) – most commonly used one for pain now • Pregabalin (Lyrica) – 2nd generation gabapentin Best in lacinating (stabbing or piercing) neuropathic pain |
|
|
What is central pain sensitization, and how does it impact pain treatment?
|
In central pain sensitization, the intensity of transmitted nociception is increased, and non-nociceptive fibers (e.g., A-beta fibers) are recruited into transmitting pain. This makes pain treatment difficult, because blockade of A-beta fibers (that now aberrantly trigger pain) cause patients to lose critical functions like touch. There are 3 suggested mechanisms of central sensitization:
1) Windup: C-fiber that fires for a prolonged period is allowed to rest. Subsequent triggering of the C-fiber results in exaggerated transmission of nociceptive impulses in the dorsal horn of the spinal cord. This results from the first, prolonged stimulus causing a postsynaptic neuron in the dorsal horn to activate its NMDA receptor, which lowers the threshold for firing of the postsynaptic pain neuron, resulting in greater transmission of nociception. 2) Central reorganization: A-beta fibers sprout synapses to nociceptive synapses in the substantia gelatinosa and thus touch can trigger pain, causing allodynia (pain with touch) 3) Central prostaglandin formation: in the presence of injury or inflammation in the body, COX-2 gets upregulated in the CNS, causing increased formation of prostaglandin E. Prostaglandin in the dorsal horn of the spinal cord does 3 things: a. Lowers firing threshold of the postsynaptic neurons transmitting nociception, contributing therefore to hyperalgesia b. Increases the release of pain neurotransmitters from C-fibers , adding to hyperalgesia c. Inhibits action of the descending inhibitory pathway, limiting actions of opioids and increasing pain transmission |
|
|
What are 2 differences between acute and chronic pain in terms of how they are defined and how they are treated?
|
Chronic pain persists despite maximal medical treatment of an underlying condition.
Acute pain serves a biologic function (e.g., self-preservation), whereas chronic pain serves no biologic function. Acute and chronic pain can co-exist. |
|
|
What’s one example of nociception without pain? What about pain without nociception?
|
• Nociception is the neurophysiologic process of transmitting signals that normally are perceived as pain.
• Nociception without pain – patient undergoing surgery with general anesthesia • Pain without nociception – phantom limb pain |
|
|
What is neuropathic pain?
|
Neuropathic pain is due to signals arising from damaged nerve fibers or brain/spinal cord damage.
|
|
|
Stenosis of what brain structure is a common cause of obstructing/noncommunicating hydrocephalus in newborns?
|
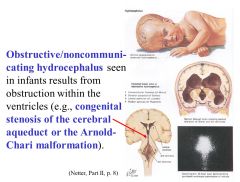
Stenosis of the cerebral aqueduct
Also, Arnold-Chari malformation can cause obstruction. |
|
|
What type of sensation is carried in the lateral corticospinal tract?
|
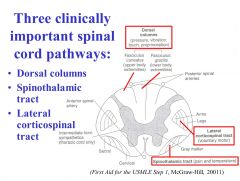
The lateral corticospinal tract carries movement commands from the contralateral motor areas of cortex.
|
|
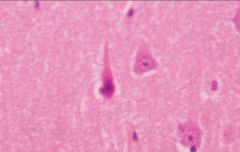
What is this, and in what circumstances would you see it?
|
• Red neuron, in response to acute neuronal injury (hypoxia/ischemia)
• appear after 12-14 hours (red neuronal change becomes more overt and diffuse after 24-48 hours and can persist for 2 weeks) |
|
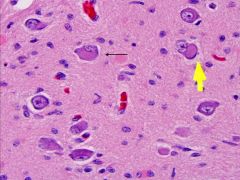
What is this, and in what circumstances would you see it?
|
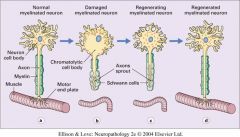
Chromatolysis is the dissolution of the Nissl bodies in the cell body of a neuron. It is an induced response of the cell usually triggered by axotomy, ischemia, toxicity to the cell, cell exhaustion, virus infections, and hibernation in lower vertebrates. Neuronal recovery through regeneration can occur after chromatolysis, but most often it is a precursor of apoptosis.
|
|
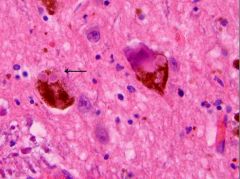
What is this, and in what circumstances would you see it?
|
Lewy body; seen in Parkinson’s Disease; (proteinopathy)
|
|
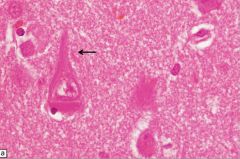
What is this, and in what circumstances would you see it?
|
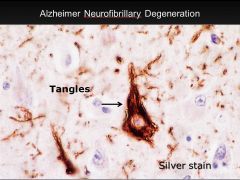
• Neurofibrillary tangles; seen in Alzheimer’s disease
• Microtubule-associated tau protein in an abnormal highly phosphorylated form; contains phosporylated tau protein. |
|
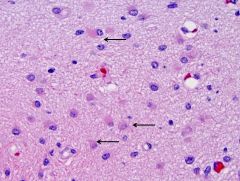
What is this, and in what circumstances would you see it?
|
• Gliosis (hypertrophy and hyperplasia of astrocytes)
• Gliosis is a nonspecific reactive change of glial cells in response to damage to the central nervous system (CNS). In most cases, gliosis involves the proliferation or hypertrophy of several different types of glial cells, including astrocytes, microglia, and oligodendrocytes. In its most extreme form, the proliferation associated with gliosis leads to the formation of a glial scar. • Gliosis has historically been given a negative connotation due to its appearance in many CNS diseases and the inhibition of axonal regeneration caused by glial scar formation. However, gliosis has been shown to have both beneficial and detrimental effects, and the balance between these is due to a complex array of factors and molecular signaling mechanisms, which affect the reaction of all glial cell types. |
|
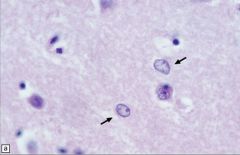
What is this, and in what circumstances would you see it?
|
• Alzheimer type II astrocytes/glia (arrows) in the putamen tend to have an enlarged, oval, vesicular nucleus and very scanty cytoplasm.
• Not related to alzheimer disease • Seein in hypperammonemic states, Wilson’s disease and liver failure |
|
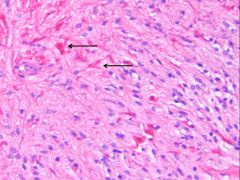
What is this, and in what circumstances would you see it?
|
• Rosenthal fibers (thick elongated, brightly eosinophilic irregular structures in astrocytic processes)
• Seen in long-standing gliosis and slow growing tumors |
|
|
Can you describe the flow of CSF?
|
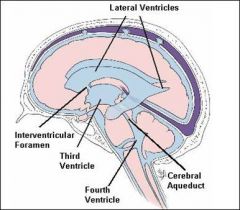
CSF is produced in the brain by modified ependymal cells in the choroid plexus (approx. 50-70%) and the remainder is formed around blood vessels and along ventricular walls. It circulates from the lateral ventricles to the foramina of Monro (Interventricular foramina), third ventricle, aqueduct of Sylvius (Cerebral aqueduct), fourth ventricle, foramen of Magendie (Median aperture) and foramina of Luschka (Lateral apertures), subarachnoid space over brain and spinal cord. It should be noted that the CSF moves in a pulsatile manner throughout the CSF system with nearly zero net flow. CSF is reabsorbed into venous sinus blood via arachnoid granulations.
|
|
|
What structures would be affected in an uncal herniation?
|
• Posterior cerebellar artery
• CNs 3 and 6 |
|
|
What does CADASIL stand for?
|
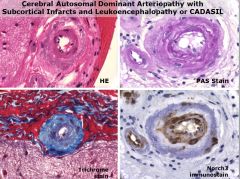
• Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy or CADASIL
|
|
|
What’s the most common cause of non-traumatic subarachnoidal hemorrhage?
|
• Rupture of saccular aneurysm
• Pathology: Loss of Internal Elastic Lamina (IEL) |
|
|
What enzyme is responsible for the synthesis of ACh?
|
ACh is synthesized from acetyl coA and choline by choline acetyltransferase.
|
|
|
Which antidepressants are selective?
|
• Selective
o SSRIs (Selective Serotonin Reuptake Inhibitors) – e.g., fluoxetine o SNRIs (Serotonin-Norepinephrine Reuptake Inhibitors) – e.g., venlafaxine • Nonselective o Typical and atypical antidepressants (e.g., desipramine or buproprion) |
|
|
What are some reasons why few peptides are useful as drugs?
|
• They’re poorly absorbed when given orally
• They have a short duration of action because of rapid degradation in vivo • They fail to cross the BBB • They’re expensive to manufacture |
|
|
What are the biogenic amines?
|
• Serotonin
• Dopamine • Melatonin • Histamine • Norepinephrine |
|
|
What is fiber type grouping?
|
Fiber type grouping is when a muscle is damaged and undergoes denervation followed by reinnervation and the random mosaic of fiber types is lost and replaced by fibers that are all of one type.
|
|
|
What is grouped atrophy?
|
Grouped atrophy is when a muscle is damaged and undergoes denervation followed by reinnervation and the motor unit is subsequently lost and a large group of atrophic fibers develop.
|
|
|
What gene is mutated in Duchenne muscular dystrophy?
|
DMD is an X-linked recessive mutation of the dystrophin gene (30% are spontaneous mutations) whereby there is near or total loss of dystrophin protein.
|
|
|
What’s the difference between Becker muscular dystrophy (BMD) and Duchenne muscular dystrophy (DMD)?
|
• BMD has a dystrophin protein that’s semifunctional and of abnormal size and/or amount, whereas dystrophin has little to no dystrophin.
• BMD clinical features are similar to DMD but onset is later and progression slower • The muscle biopsy for BMD is similar to DMD but less severe |
|
|
What disease is an autosomal recessive disorder caused by mutation of lysosomal acid alpha-glucosidase, and what are its clinical features?
|
• Acid Maltase deficiency (Pompe) Disease is an autosomal recessive disorder caused by mutation of lysosomal acid alpha-glucosidase
• Clinical features: o Infantile: generalized weakness and hypotonia o Adult: respiratory and limb-girdle weakness |
|
|
What disease is characterized by exercise intolerance in childhood and exertional pain with brief intense (anerobic activities) as well as myoglobinuria?
|
Myophosphorylase deficiency (McArdle’s) Disease is an autosomal recessive disorder caused by mutation of gene that encodes myophosphorylase. Abnormal enzyme leads to impaired glycolysis during anaerobic activity and glycogen accumulation. Intracellular ADP is increased and inorganic phosphate is reduced, which may inhibit dissociation of the acitn-myosin cross-bridge.
|
|
|
What are MPZ and PMP?
|
MPZ and PMP are essential proteins of compact myelin
- MPZ: Compaction of myelin - PMP: Cell cycle proliferation and apoptosis, myelin regulation |
|
|
Can you describe some differences between type 1 and type 2 muscle fibers?
|
• Type 1 for sustained force and weight bearing activity; good at low-impact fatigue resistant activities e.g., posture (don’t require a lot of force but need to be sustained for a long time) -> greater blood supply and more mitochondria
• Type 2 for sudden movements; fatigue very quickly but can generate a lot of power. Not a lot of blood/vascular supply. A lot of glycolytic enzymes • Remember, the motor unit determines the fiber type and all fibers of a single motor unit are of the same type. Distribution is random. |
|
|
What myopathy is characterized by proximal weakness and skin involvement (e.g., erythematous rash on eyelids, knucles, chest)?
|
Dermatomyositis
|
|
|
How does the immune response to polymyositis differ from that of the one to dermatomyositis?
|
The immune response to polymyositis is cell-mediated (i.e. involves activation of macrophage, NK cells, antigen specific cytotoxic T lymphocytes and the release of various cytokines), whereas the one to dermatomyositis is humoral immune system mediated with immune complex and complement deposition around capillaries in muscle.
|
|
|
What is myotonia?
|
Myotonia is difficulty relaxing after forceful contraction (e.g., sprinting and after taking the first step you collapse, because you can’t relax the muscle).
|
|
|
What is the pathogenesis of myotonic dystrophy?
|
• Two genetically distinct forms of myotonic dystrophy
– Myotonic dystrophy type 1(DM1) – Myotonic dystrophy type 2 (DM2) • Pathogenesis: – DM1: CTG trinucleotide expansion of the myotonin protein kinase gene resulting in disruption of multiple target proteins including chloride ion channels • Abnormal sarcolemmal excitability • Ion channel abnormalities lead to current amplitude and channel gating changes, and eventually channel inactivation – DM2: CCTG expansion of zinc finger protein 9 • Severity of myopathy correlates with size of expansion – Each generation has more severe presentation and earlier disease onset (anticipation) |
|
|
What disease has fluctuating weakness that gets worse with sustained activity, extraocular muscle weakness or ptosis present initially in 50% of patients and has proximal weakness greater than distal limb weakness?
|
Myasthenia gravis.
Further, thymus abnormalities are noted in about 75% of patients (hyperplasia in 85% of cases and thymoma in 15%) |
|
|
In what neuromuscular junction disorder must you look aggressively for lung carcinoma?
|
Lambert-Eaton syndrome has paraneoplastic syndrome in 2/3rds of cases (90% small cell lung carcinoma)
• Clinical features: – Proximal weakness in leg and arm muscles – Ocular & bulbar symptoms mild – Strength may initially improve after exercise • Labs: Voltage gated Ca channel Ab • Treatment: – Treat malignancy – Immuosuppression and/or plasma exchange as in MG |
|
|
What are the clinical features and pathophysiology of botulism?
|
• Pathophysiology: Exotoxin of C. botulinum interferes with presynaptic Ach release by preventing ACh vesicles from docking to motor nerve terminal
• Clinical features: – Symptoms appear 12 to 36 hours after ingestion, evolve over several days – Ptosis and extraocular muscle weakness – Bulbar muscles usually affected causing dysphagia and dysarthria – Weakness typically diffuse and usually symmetric (proximal > distal) |
|
|
By when is the risk of disease for multiple sclerosis MS acquired?
|
Risk for disease in MS is acquired by adolescence (~15 y/o) and maintained with migration, which suggests environmental exposure may have a role in pathogenesis
|
|
|
Are there any genetic loci that are associated with multiple sclerosis (MS)?
|
• HLA DR 2*1501 has been identified in northern Europeans
|
|
|
How would you define a multiple sclerosis exacerbation?
|
• Neurological disturbance lasting at least 24 hours and occurring in the absence of fever or infection. Often progresses over hours to days, but can occur abruptly.
• By definition any symptoms occurring within 30 days is considered one episode. • Rarely individuals do not have attacks, but gradual progression of symptoms over time. |
|
|
What are the 2 most common presenting symptoms for multiple sclerosis, and what are some other symptoms?
|
2 most common:
• Sensory symptoms (e.g., numbness, tingling, burning, tightness) • Visual loss/optic neuritis (progressive monocular visual loss, impaired color vision, +/- pain with eye movement, controcecal scotoma (an area of partial alteration in the field of vision, consisting of a partially diminished or entirely degenerated visual acuity that is surrounded by a field of normal or well-preserved vision) Other symptoms: • Motor disturbance • Brainstem cerebellar o Vertigo, diplopia |
|
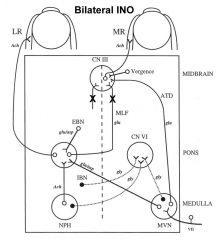
What disease is bilateral INO (Internuclear ophthalmoplegia) classically pathognomonic for?
Internuclear ophthalmoplegia is a disorder of conjugate lateral gaze in which the affected eye shows impairment of adduction. When an attempt is made to gaze contralaterally (relative to the affected eye), the affected eye adducts minimally, if at all. The contralateral eye abducts, however with nystagmus. Additionally, the divergence of the eyes leads to horizontal diplopia. That is, if the right eye is affected the patient will "see double" when looking to the left, seeing two images side-by-side. Convergence is generally preserved. |
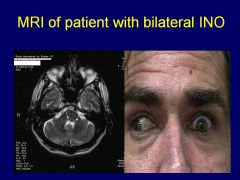
Multiple sclerosis
|
|
|
True/False: multiple sclerosis patients can experience brain atrophy?
|
• True; brain atrophy occurs in MS at a rate of 0.6% to 1%/year
• Cause is unknown • Relationship between lesions and atrophy is only moderate • New evidence suggests atrophy and disability are related |
|
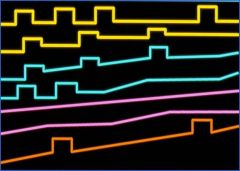
Can you match the pattern with the clinical title for the pattern (Relapsing-remitting, Secondary progressive, Primary progressive, Progressive relapsing)?
|
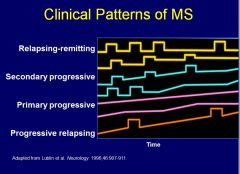
See image – listed in order of incidence
|
|
|
How would you treat an acute multiple sclerosis exacerbation?
|
• 1 gm IV methylprednisolone for 3-5 days
• Plasma exchange for severe attacks in which there is no response to steroids |
|
|
Which of the clinical patterns for multiple sclerosis does not have a defined treatment for it?
|

Primary Progressive does not have a defined treatment schema
|
|
|
What’s the unique factor about interferon-beta treatment for multiple sclerosis (MS)?
|
Interferons work at the BBB, that’s what’s unique about it
|
|
|
Why is mitoxantrone not used for multiple sclerosis any longer?
|
• Mitoxantrone is a chemotherapeutic with broad activity
• Big issues with mitoxantrone = cardiac dysfunction (why it’s not used anymore) • Also causes amenorrhea/infertility and a host of other side effects |
|
|
What’s an important side effect concern with Natalizumab?
|
Natalizumab can cause progressive multifocal leukoencephalopathy (PML -- for which there is no treatment)
|
|
|
What’s an important side effect concern with Teriflunomide?
|
Teriflunomide can get into the semen, which is important for family planning
|
|
|
Should you give someone with MS a vaccine?
|
• Avoid vaccines in the setting of people with a relapse
• Clear increased exacerbation risk in the peri-infectious period • Influenza, Hepatitis B, and Tetanus appear to be safe • Delay vaccinations during significant relapses and avoid live vaccines |
|
|
What is the difference between neuromyelitis optica (NMO) and multiple sclerosis (MS)?
|
NMO is also a demyelinating disease that results in attacks of optic neuritis and myelitis. Spinal cord lesions are longitudinally extensive with greater than 3 vertebral segments. A serological marker is found in NMO directed against a water channel called aquaporin-4. NMO is thought to be antibody-mediated whereas MS auto-reactivity is mediated by T cells.
|
|
|
What signs/symptoms should make one suspect a leukodystrophy, and what additional diagnostic testing is necessary/helpful?
|
• Leukodystrophies, in general, present in early childhood with psychomotor arrest and regression, spasticity, and ataxia, with variable peripheral nervous system involvement.
• Age of onset, gender of child, MRI appearance, and additional associated symptoms like adrenal insufficiency in adrenoleukodystrophy can point towards a specific leukodystrophy • Treatment is generally only supportive |
|
|
What is Krabbe disease?
|
• Krabbe disease is a leukodystrophy
• Autosomal recessive disorder resulting from deficiency of galactocerebrosidase, with resultant formation of globoid cells in white matter. • Children are normal at birth, but then develop symptoms around 3-6 months of age with irritability, spasticity, feeding difficulties, and slowed developmental progress. • Later, psychomotor regression, seizures, increased tone and extensor posturing occurs, culminating in death around 2 years of age. |
|
|
What is metachromatic leukodystrophy?
|
• Autosomal recessive disorder of myelin metabolism caused by deficiency of arylsulfatase A enzyme.
• Infantile (most common), juvenile, and adult forms • Normal early development with progressive gait abnormalities beginning around 2-4 years of age with spasticity, ataxia, or distal weakness of the feet with loss of reflexes (concomitant peripheral neuropathy). • Later, children become bedridden with spastic quadriplegia, feeding difficulties, and optic atrophy, with death within the first decade of life. |
|
|
What is adrenoleukodystrophy (ALD)?
|
• X-linked recessive disorder with variable expressivity resulting from the inability to catabolize very-long-chain fatty acids (VLCFAs) within peroxisomes. There is a loss of myelin with gliosis and lymphocytic infiltration.
• Adrenomyeloneuropathy (AMN) o Typically presents in early adulthood with spastic paraparesis, peripheral neuropathy, and adrenal insufficiency. o MRI abnormalities may occur even in those without signs/symptoms of cortical involvement. |
|
|
What is Pelizaeus-Merzbacher Disease?
|
• X-linked recessive disease caused by defective biosynthesis of a myelin sheath protein, linked to the proteolipid protein (PLP) gene.
• Presents in infancy or early childhood with pendular nystagmus, choreoathetosis, hypotonia, psychomotor arrest then regression. • Death by 5-7 years of age. |
|
|
What is Canavan Disease (Spongy Degeneration of the Nervous System)?
|
• Autosomal recessive disease caused by deficiency of aspartoacylase.
• Presents with psychomotor arrest and regression within the first 6 months of life, with macrocephaly noted. Optic atrophy with eventual blindness occurs around 6-10 months. • Diagnostic features: o increased N-acetylaspartic acid (NAA) in urine and plasma and low aspartoacylase activity in fibroblasts o MRI brain: diffusely increased lucency of the WM with poor demarcation of gray and white matter o NCV and CSF studies are typically normal • Treatment: symptomatic |
|
|
What is Alexander Disease?
|
• Rare disorder caused by mutations in the glial fibrillary acid protein (GFAP) resulting in diffuse accumulation of Rosenthal fibers.
• Onset anytime from birth to early childhood, and characterized by megalencephaly, psychomotor regression, spasticity, and seizures. • Diagnostic features: MRI demonstrates progressive leukodystrophy with frontal predominance. Absence of optic atrophy helps differentiate from Canavan. • Treatment: symptomatic |
|
|
What is Vanishing White Matter Disease?
|
• Autosomal recessive disease with mutations in one of several genes that leads to loss of eIF2B function, which is important for protein synthesis and regulation under stress conditions.
• Initial normal develop with chronic deterioration and cerebellar ataxia, with acute worsening triggered by intercurrent illness, fever, minor head trauma, and fright. |
|
|
What is Meniere’s disease?
|
Episodic vestibular imbalance with hearing disturbance
|
|
|
What brain system/reflex allows us to see while the head is moving?
|
VOR – Vestibular Ocular Reflex
|
|
|
What is the most common cause of recurrent vertigo?
|
• BPPV – Benign Paroxysmal Positional Vertigo is the most common and treatable cause of dizziness
• BPPPV is a disorder arising in the inner ear. Symptoms are repeated episodes of positional vertigo, a spinning sensation caused by changes in the position of the head. • Can diagnose with the Dix-Hallpike test where a positive test results in nystagmus upon bringing a patient to a lying position from a sitting position with their head turned |
|
|
What is the pathophysiology behind vertigo?
|
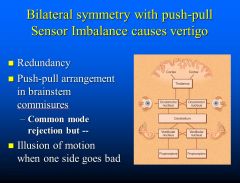
Push-pull sensor imbalance causes vertigo
|
|
|
How would you manage a patient with Meniere’s disease?
|
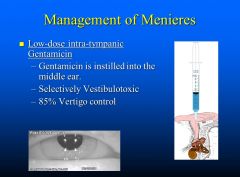
Low dose intra-tympanic gentamicin (85% vertigo control)
|
|
|
What are some migraine prophylactic treatments?
|
• Anticonvulsants
o Topiramate – weight loss, word finding, teratogen o Sodium valproate – tremor, teratogen • Blood pressure lowering drugs o Verapamil – constipation o Beta-blockers – lowers BP, depression • Antidepressants (low dose, not all) o Tricyclics – sedation and weight gain o Venlafaxine –few side effects withdrawal |
|
|
What is the definition of a seizure?
|
Seizure: the clinical manifestation of an abnormal and excessive excitation and synchronization of a population of cortical neurons
|
|
|
What is the definition of epilepsy?
|
Epilepsy is a predisposition to unprovoked seizures.
|
|
|
What’s the difference between a simple and a complex partial seizure?
|
If consciousness is retained, it’s a simple partial seizure; if it’s impaired, it’s a complex partial seizure
|
|
|
What characteristic EEG abnormalities are shown in patients with epilepsy?
|
Spikes/sharp waves
|
|
|
What characteristic EEG abnormalities are shown in patients with childhood absence epilepsy?
|
Generalized 3Hz spike and wave
|
|
|
What are important triggers for seizures?
|
• Sleep deprivation
• Stress • Alcohol |
|
|
What are adverse effects for phenytoin?
|
• Drowsiness
• Ataxia • Confusion • Insomnia • Nystagmus • Gum hyperplasia • Hirsutism |
|
|
What are adverse effects for carbamazepine?
|
• Agranulocytosis and aplastic anemia
• Mild hyponatremia |
|
|
What are drug interactions for carbamazepine?
|
Stimulates metabolism of other drugs by inducing enzymes (e.g., chemotherapy, Coumadin)
|
|
|
What is the mechanism of action of benzodiazapines?
|
Benzodiazapines are allosteric modulators of GABA-A receptors and increase the frequency of chloride channel opening
|
|
|
What is the mechanism of action of benzodiazapines?
|
Benzodiazapines are allosteric modulators of GABA-A receptors and increase the frequency of chloride channel opening
|
|
|
What is the first line drug for generalized epilepsy, and what are its adverse effects?
|
• Valproate (Valproic acid) is a first line drug for generalized epilepsy.
• Hepatotoxicity is rare but severe • Causes birth defects o Neural tube defects (e.g., spina bifida) |
|
|
What drug can be used to treat absence seizures, and what is its mechanism of action?
|
• Ethosuximide has a narrow indication for the specific treatment of absence seizures.
• It blocks T-type Ca++ currents in the thalamus. |
|
|
What is an important side effect of oxcarbazepine?
|
• Oxcarbazepine is an improved version of carbamazepine for monotherapy in partial seizures
• Important adverse effect: Prominent hyponatremia |
|
|
What potent broad spectrum AED can be used as a monotherapy and adjunctive therapy for generalized and focal epilepsies and causes Steven Johnson Syndrome?
|
Lamotrigine-1; safe in pregnancy
Lamotrigine-2 is also safe in pregnancy |
|
|
What are important adverse effects of Felbamate?
|
• Felbamate is a 3rd line drug for refractory partial seizures, very potent, broad spectrum, rarely used due to severe potential toxicity
• Adverse effects: aplastic anemia and severe hepatitis |
|
|
What’s an important adverse effect of Levetiracetam?
|
It can cause irritability
|
|
|
What are important adverse effects of Topiramate/Topamax “Dopamax?”
|
• Topiramate is a potent broad spectrum AED used as monotherapy and adjunctive therapy for partial seizures and generalized epilepsies
• Adverse effects: o Memory and cognitive effects (makes you stupid) o Causes significant appetite suppression and weight loss • Effective for migraine prophylaxis and also treats neuropathic pain and tremor |
|
|
What is the site of action of Lyrica/Pregabalin, and what are adverse effects?
|
• Acts at an auxiliary subunit of voltage-gated Ca++ channels. Reduces the synaptic release of several neurotransmitters by binding alpha2-delta subunits and reducing neuronal calcium currents, which decreases neuronal excitability and seizures.
• Causes significant appetite increase and weight gain |
|
|
What important drugs may exacerbate epileptic seizures?
|
• Tramadol (analgesic)
• Venflaxine (antidepressant) |
|
|
What are the important alternative uses for AEDs?
|
• Neuropathic pain: Gabapentin, carbamazepine, pregabalin
• Bipolar disorder: lamotrogine, valproate • Migraine: valproate, topiramate, zonisamide |
|
|
What organisms are most commonly responsible for community-acquired bacterial meningitis?
|
• Streptococcus pneumonia
• Neisseria meningitides • Group B Streptococci • Listeria monocytogenes • Haemophilus influenza |
|
|
What is the most common pathogenesis (i.e., method of spread) of CNS infections?
|
Hematogenous transmission (e.g., nasopharyngeal colonization w/ Neisseria meningitides acaquired by inhalation of aerosolized droplets from an asymptomatic carrier). The same is true for encephalitis in terms of hematogenous transmission being the most common route of transmission.
|
|
|
In the diagnosis of meningitis, what would be the CSF parameters (opening pressure, WBC count/predominant cell type, protein, glucose) for the following cause of meningitis: bacterial, viral, and TB/fungal meningitis?
|
• Bacterial
o Opening pressure: >250 mm H2O o WBC count > 1000 (>50% neutrophils) o Protein > 100 o Glucose < 100 • Viral o Opening pressure: normal or mildly elevated (normal = 50-180 mm H2O) o WBC count < 500 (lymphocytes; may see neutrophils early) o Protein < 100 o Glucose > 40 • TB/fungal o Opening pressure > 250 mm H2O o WBC count 100 – 500 (lymphocytes) o Protein > 100 o Glucose < 40 |
|
|
What is the classic triad for viral meningitis?
|
• Fever
• Headache • Photophobia |
|
|
What are the 3 main symptoms for the clinical presentation of encephalitis?
|
• Fever
• Headache • Altered level of consciousness (decreased mentation early vs later development in meningitis) |
|
|
What organism/group of organisms is the most common cause of brain abscess?
|
Streptococci (aerobic, anaerobic, and microaerophilic)
|
|
|
What are the 3 main symptoms for the clinical presentation of brain abscess?
|
• Fever
• Headache • Focal neurologic deficit (present in < 50% of cases) |
|
|
How does a spinal epidural abscess present clinically?
|
• Fever
• Back pain • Progresses to paresis and/or bowel/bladder dysfunction • Untreated, may rapidly progress to paraplegia |
|
|
What are normal levels for CSF WBCs, protein, glucose?
|
• WBC: 0-10
• Protein: 15-50 • Glucose: 40-80 |
|
|
What factors are associated with risk of death in meningitis?
|
• Decreased level of consciousness on admission
• Seizures • Signs of increased intracranial pressure • Extremes of age (infants or age>50) • Delayed treatment |
|
|
CNS infection with which virus of the following two has a better prognosis :HSV-1, HSV-2?
|
HSV-2 has the better prognosis (i.e., benign prognosis), whereas HSV-1 can cause encephalitis and is associated with a poor prognosis
|
|
|
What are the causes of encephalitis that Flaherty suggested were important in his lecture?
|
• Viral
o HSV o West Nile o HIV • Non-viral o Treponema pallidum |
|
|
What virus causes progressive multifocal leukoencephalopathy?
|
• JC virus seropositivity 60%-80%
• Progressive multifocal leukoencephalopathy – Infection of oligodendrocytes, basophilic intranuclear inclusions – Occurs only in setting of severe immunosuppression – CSF-PCR: 60%-100% sensitive • Treatment – No specific treatment – Treat HIV, reduce immunosuppression |
|
|
What is the definition of an ischemic penumbra?
|
An ischemic penumbra is an area of the brain that will ultimately infarct.
|
|
|
What is the duration of time after an ischemic stroke in which the administration of tPA will improve the outcome?
|
Intravenous tPA in acute ischemic stroke up to 4.5 hours from symptom onset improves outcome from stroke.
|
|
|
What was the main finding of the DEFUSE2 study of ischemic strokes?
|
• Patients with a Target Mismatch respond more favorably to endovascular reperfusion therapy than patients without a Target Mismatch.
• 5-fold increase in good outcome in patients with mismatch. 5-fold reduction in good outcome in people without mismatch (they’re exposed only to toxicity of therapy without any of the benefit) |
|
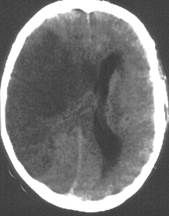
What radical treatment has proven efficacy in patients with MCA infarction?
|
• Decompressive hemicraniectomy helps
• Pts with huge infarct at risk for developing huge edema with midline shift would have neurosurgeons remove a huge part of the skull, removing the dura, allowing the brain to swell out of the skull (which isn’t great) instead of allowing the brain to move the midline and crush the midbrain (which is worse), and they would replace the skull once the swelling had gone down • It’s the most effective therapy in stroke neurology • These people don’t end up going back to normal; they’re left severely disabled, but they’re transferred from the category of dead to generally independence/needing some help |
|
|
What is the only (common) stroke mechanism we treat with warfarin (others are treated with antiplatelet agents--with a few rare exceptions)?
|
Atrial fibrillation
|
|
|
What is the most common location of cerebral amyloid angiopathy?
|
• Cerebral amyloid angiopathy (CAA) is a form of angiopathy in which amyloid deposits form in the walls of the blood vessels of the CNS.
• Lobar is most common location • Often associated with Alzheimer’s Disease |
|
|
Which type of intracranial hemorrhage patients should NEVER receive warfarin?
|
Cerebral amyloid angiopathy patients should NEVER receive warfarin (even if they have atrial fibrillation).
|
|
|
If a previously stable patient on warfarin suddenly develops new neurological symptoms, what may you suspect?
|
Cerebral bleed; until proven otherwise
|
|
|
What tumors are notorious for bleeding?
|
• Lung
• Breast • Kidney • Melanoma |
|
|
Why do younger patients fare worse than older patients with increased intracranial pressure?
|
Younger patients fare worse, because older patients have a little brain atrophy, so their brains can be displaced more.
|
|
|
How does MRI fare vs CT for acute and subacute/chronic intracranial hemorrhage (ICH)?
|
• Acute ICH: CT=MRI
• Subacute/chronic ICH: MRI>CT (more sensitive) |
|
|
How important is the cerebral perfusion pressure (CPP) to preventing cerebral damage?
|
You must keep the CPP above 70 mm Hg to prevent cerebral damage.
|
|
|
What is the imaging study of choice for intracranial epidural hematoma?
|
Noncontrast CT scanning of the head – not only visualizes skull fractures but also directly images an epidural hematoma
|
|
|
What is the imaging study of choice for intracranial subdural hematoma?
|
Noncontrast head CT scan
|
|
|
What structure is responsible for inducing ectoderm to become future neural tube?
|
Notochord
|
|
|
When should women of child-bearing age take folic acid and why?
|
• Before pregnancy to prevent neural tube defects
• At least 28 days before conception and continued for the first 2 months of pregnancy |
|
|
By when does ectoderm differentiate into the neural plate in humans?
|
By ~ day 17
|
|
|
By when do the anterior and posterior neuropores close?
|
• Anterior neuropore – 24 days
• Posterior neuropore – 27 days |
|
|
What is the “inside out” establishment of the cerebral cortex?
|
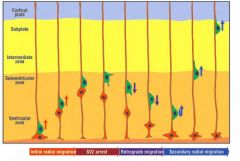
Cells that are born early stay near the ventricle, and cells born later end up on top of the early cells.
|
|
|
What are clinical sequelae of cortical dysplasia?
|
• Cortical dysplasia is a congenital abnormality where the neurons in an area of the brain failed to migrate in the proper formation in utero.
• Seizures are usually the only clinical sequelae |
|
|
Which grade of cortical dysplasia tends to get better after surgery, grade I or II?
|
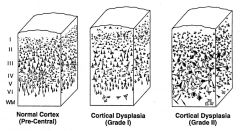
Grade II
|
|
|
What genes are mutated in lissencephaly?
|
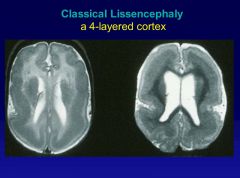
• LIS1 and DCX mutations account for 80% of typical cases
• Both are associated with microtubules |
|
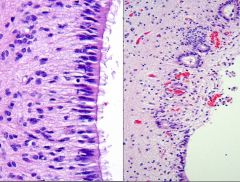
What process is visualized here, and what causes it?
|
• Conversion from normal ependymal to ependymal granulations
• Reaction to inflammation or hemorrhage in the ventricles • Disruption of ependymal lining with proliferation of subependymal astrocytes and formation of pinched off small ependymal canals |
|
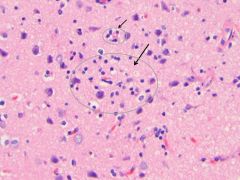
What process is visualized here, and what causes it?
|
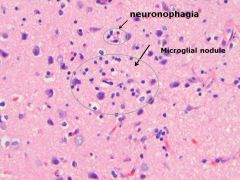
• Microglial nodules, aggregates of microglia and lymphocytes around microscopic necrosis
• Around dying neurons, neuronophagia • Seen in response to injury or infection. |
|
|
What are UMN lesion signs?
|
Weakness
Hyperreflexia Increased tone Babinski Spastic paralysis Clasp knife spasticity |
|
|
What are LMN lesion signs?
|
Weakness
Atrophy Fasciculation Hyporeflexia Decreased tone |
|
|
Which cranial nerve nuclei are in the medulla? Pons? Midbrain?
|
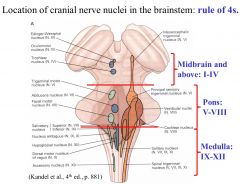
Medulla: 9, 10, 11, 12
Pons: 5, 6, 7, 8 Midbrain: 1, 2, 3, 4 |
|
|
What are the indications of use for benzodiazepines (e.g., Midazolam)?
|
• Sedation
• Hypnosis • Amnesia • Anxiolysis |
|
|
What is the only anesthetic that is antiemetic?
|
Propofol
|
|
|
How does propofol drop the BP of a pt?
|
Decreases SVR
|
|
|
What is the indication of use for barbiturates (e.g., thiopental)?
|
Induction of general anesthesia
|
|
|
What are the components of myocardial O2 supply and demand?
|
• Demand
o HR o Contractility o Afterload o Preload • Supply o HR o O2 content o Vascular tone o CPP (Coronary Perfusion Pressure) |
|
|
What is the mechanism of action (MOA) of barbirutrates like thiopental? What are important adverse effects?
|
• MOA: Barbiturates increase the duration of Cl- conductance leading to neuronal hyperpolarization. The drug itself activates the receptor (even if you didn’t have GABA around, it would induce the opening of the GABA channel)
• Adverse effects: o myocardial depression and resulting hypotension o decreases cerebral metabolic rate of O2 consumption (CMRO2) and simultaneously cerebral blood flow |
|
|
What is the mechanism of action of propofol? What are important adverse effects?
|
• MOA: Propofol increase the duration of Cl- conductance leading to neuronal hyperpolarization.
• Adverse effects: o Severe hypotension (due to loss of systemic vascular resistance and peripheral vasodilation) o Central medullary respiratory depression o decreases cerebral metabolic rate of O2 consumption (CMRO2) and simultaneously cerebral blood flow |
|
|
What is the mechanism of action of etomidate? What are important adverse effects?
|
• MOA: Etomidate increase the duration of Cl- conductance leading to neuronal hyperpolarization.
• Confers cardiovascular stability during use o Pt’s blood pressure more likely to remain in normal range • Adverse effects: o Central medullary respiratory depression o decreases cerebral metabolic rate of O2 consumption (CMRO2) and simultaneously cerebral blood flow |
|
|
What is the mechanism of action of benzodiazepines like Midazolam? What are important adverse effects?
|
• MOA: Benzodiazepines increase the frequency of opening of Cl- channel opening induced by GABA
• Benzodiazepines produce amnesia • Adverse effects: o Central medullary respiratory depression o decreases cerebral metabolic rate of O2 consumption (CMRO2) and simultaneously cerebral blood flow |
|
|
What drug can be used to accelerate the recovery from depression due to benzodiazepine overdose?
|
Flumazenil is an antagonist of the benzodiazepine binding site on the GABA-A receptor
|
|
|
What is the mechanism of action of ketamine? What are important adverse effects?
|
• MOA: Ketamine produces dissociative anesthesia resulting in catatonia, amnesia, and analgesia by inhibiting the NMDA receptor.
• Adverse effects: o Post-operative psychic phenomena o Sympathomimetic properties: increases HR and BP (thus contraindicated in pts w/ intracranial HTN) o Increases cerebral blood flow via vasodilating action o Potent bronchodilator (useful in asthmatic patients) o Potent sialogogue and increases salivation o Induces profound nystagmus |
|
|
What is the mechanism of action of nitrous oxide? What are important adverse effects?
|
• MOA: inhibits the NMDA receptor (no effect on GABA-A receptor)
• Adverse effects: (all at high concentrations) o N2O causes bone marrow depression and hematopoietic malfunctions (megablastic anemia, leukopenia, thrombocytopenia) o Can irreversibly oxidize the cobalt atom in vit B12, and vit B12 deficiency affects myelin formation o Spontaneous abortions or congenital anomalies have been reported for women with chronic exposure to N2O |
|
|
Which general area of the hypothalamus is responsible for sleep? Wakefulness?
|
o Anterior hypothalamus is responsible for sleep
o Posterior hypothalamus is responsible for wakefulness |
|
|
What neurons are responsible for promoting and maintaining wakefulness?
|
Hypocretin-containing neurons help promote and maintain wakefulness.
|
|
|
What neurons play a large role in REM sleep?
|
Cholinergic nuclei located near the pedunculopontine (PPT) and laterodorsal tegmental (LDT) nuclei
|
|
|
What areas of the brain help in turning off REM sleep?
|
• Tuberomammillary Nucleus (histamine)
• Ventral tegmentum (dopamine) • Dorsal raphe (serotonin) • Locus Coeruleus (epinephrine) |
|
|
What neurotransmitters are increased/decreased during wakefulness?
|
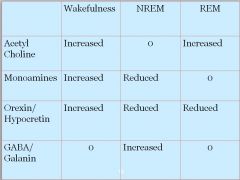
• Acetylcholine increased
• Monoamines increased • Orexin/Hypocretin increased |
|
|
What neurotransmitters are increased/decreased during NREM sleep?
|
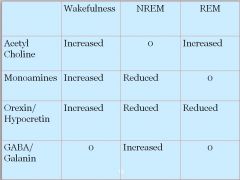
• Only GABA/Galanin increased
• Monoamines reduced • Orexin reduced |
|
|
What neurotransmitters are increased/decreased during REM sleep?
|
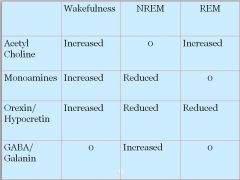
• Acetylcholine increased
• Orexin reduced • Monoamines 0 • GABA 0 |
|
|
Studies suggest what neuro receptors may be responsible for restless leg syndrome (RLS) pathology?
|
Brain imaging studies suggest reduced D2 receptor activity.
|
|
|
How would you treat restless leg syndrome (RLS)?
|
• First line – Dopaminergic agonists
o Levodopa/ carbidopa o Pramipexole o Ropinorole • Side effects: o Rebound o Augmentation o Daytime sleepiness- D3 receptor action o Compulsive behavior such as gambling. • If you’re concerned about ICDs, you can use Gabapentin (anticonvulsant) • You could also use Opiods or benzodiazepines |
|
|
What sleeping disorder is characterized by “REM-like” phenomena during wakefulness(e.g., cataplexy, sleep paralysis, hypnopompic hallucinations)?
|
Narcolepsy
|
|
|
Loss of what neurons is thought to be the cause of narcolepsy?
|
The cause of narcolepsy is the loss of hypocretin-containing neurons
|

