![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
63 Cards in this Set
- Front
- Back
|
An advantage of fluorine
|
Advantages
- used to make plastics such as PTFE - used to make HCFCs - added to toothpaste |
|
|
A disadvantage of fluorine
|
Highly reactive
Handing must be kept to a minimum |
|
|
An advantage of chlorine
|
Water treatment, to make pesticides, medicines and bleach
|
|
|
A disadvantage of chlorine
|
Pesticides can accumulate in the environment
CFCs destroy stratospheric ozone |
|
|
An advantage of bromine
|
used in the manufacture of flame retardants, agricultural fumigants and in photography
|
|
|
A disadvantage of bromine
|
Organic bromine compounds can destroy ozone in the stratosphere
|
|
|
An advantage of iodine
|
Used in antiseptics, germicides and dyes
Iodine-131 used to diagnose thyroid diseases |
|
|
What structure is the lattice of sodium chloride
|
Simple cubic
|
|
|
Water hydrating a negative ion
|
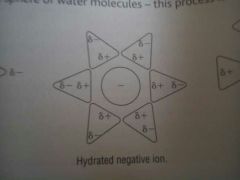
|
|
|
How do you obtain an ionic equation for a reaction?
|
Cross out all of the spectator ions(those which are the same on the left and right of the arrow)
|
|
|
What is the ionic equation for all acid/alkali neutralisation reactions?
|
H+ (aq) + OH- (aq) ---> H2O (l)
|
|
|
How do you convert cm3 to dm3?
|
÷1000
|
|
|
What are the equations involving c, v and n
|
c=n÷v n=c×v v=n÷v
|
|
|
Define the first ionisation enthalpy in words
|
The first ionisation enthalpy is the energy needed to remove one electron from each of one mole of isolated gaseous atoms if an element
|
|
|
What is the equation for the first four ionisation enthalpies
|
X(g) ---> X+(g) + e-
X +(g) ---> X 2+(g) + e- X 2+(g) ---> X 3+(g) + e- X 3+(g) ---> X 4+(g) + e- |
|
|
How does the first ionisation enthalpy vary down a group and why?
|
-Decreases
-The outermost electron is further from the nucleus -Electron shielding |
|
|
What is a redox reaction?
|
When an oxidation reaction and reduction reaction occur simultaneously
|
|
|
What is oxidation?
|
The loss of electrons
|
|
|
What is reduction?
|
The gain of electrons
|
|
|
The oxidation state of F, O, H and Cl rarely change, what are they?
|
F = -1
O = -2 H = +1 Cl = -1 |
|
|
Why do some compounds contain Roman numerals in brackets?
|
some compounds contain elements that can exist in more than one oxidation state, when this occurs the systematic name for the compound includes the oxidation state, for example FeO is iron(II) oxide
|
|
|
2Na + Cl2 ---> 2NaCl
What is the reduction half equation? |
Cl2 + 2e- ---> 2Cl-
|
|
|
2Na + Cl2 ---> 2NaCl
What is the oxidation half equation? |
2Na ---> 2Na+ + 2e-
|
|
|
When does a displacement reaction occur?
|
When a more reactive halogen like Cl2 is passed into a solution of less reactive halide ions such as Kl
|
|
|
Appearance and state of Fluorine at room temperature and it's solubility in water
|
-Pale yellow gas
-soluble in water |
|
|
Volatility and solubility in organic solvents for Fluorine
|
-Gas
-Soluble in organic solvents |
|
|
Appearance and state of Chlorine at room temperature and it's solubility in water
|
-Green gas
-Slightly soluble to give pale green solution |
|
|
Volatility and solubility in organic solvents for Chlorine
|
-Gas
-soluble to give a pale green solution in organic solvents |
|
|
Appearance and state of Bromine at room temperature and it's solubility in water
|
-Dark red liquid
-Slightly soluble to give a red-brown solution |
|
|
Volatility and solubility in organic solvents for Bromine
|
-Liquid quickly forms a brown gas on warming
-Soluble to give a red solution |
|
|
Appearance and state of Iodine at room temperature and it's solubility in water
|
-Shiny black solid
-Barely soluble, gives a brown solution |
|
|
Volatility and solubility in organic solvents for Iodine
|
-Sublimes on warming to give a purple vapour
-Soluble to give a violet solution |
|
|
Does reactivity increase or decreases down a group?
|
Decreases
|
|
|
Chlorine, Bromine, and Iodines precipitate colours
|
-Chlorine, white precipitate of silver chloride
-Bromine, cream precipitate of silver bromide -Iodine, yellow precipitate of silver iodine |
|
|
Shells are divided into sub shells called...
|
s, p, d and f
|
|
|
How many electrons can sub shell s hold?
|
2
|
|
|
How many electrons can sub shell p hold?
|
6
|
|
|
How many electrons can sub shell d hold?
|
10
|
|
|
How are the orbitals filled?
|
The orbitals are filled in order of increasing energy
|
|
|
If there are more than one orbital at the same energy level what happens?
|
The orbitals are first occupied by one electron
|
|
|
When does a dipole occur?
|
when a molecule(or part of a molecule) has a positive end and negative end
|
|
|
What makes an instantaneous dipole?
|
If the electron density is unevenly distributed at any one time, because the distribution is always changing the polarity will change
|
|
|
What makes a permanent dipole?
|
When there is a big enough difference in electronegativity, the slightly positive end attracts the slightly negative end
|
|
|
What is electronegativity?
|
The degree to which an atom of an element attracts electrons
|
|
|
Generally where are the more electronegative elements?
|
Towards the top right of the Periodic Table
|
|
|
Do C-Hal bond strength increase or decrease down a group and why?
|
-Decreases down the group
-As the atom size increases |
|
|
Does the reactivity increase or decrease down a group?
|
-Increases down the group
-As the bonds become easier to break |
|
|
What are the conditions for homolytic fission?
|
Gas phase with high temperatures or the presence of UV radiation
|
|
|
What are the conditions for heterolytic fission?
|
Dissolved in a polar solvent such as an ethanol/water mixture
|
|
|
What is a nucleophile?
|
They have one or more lone pairs of electrons that they can donate to form new bonds
|
|
|
What are two example of a nucleophile?
|
Just three examples
- hydroxide ion -water -ammonia |
|
|
what are the conditions needed to react ammonia with a halogenoalkane?
|
Halogenoalkane is heated with concentrated ammonia solution in a sealed tube
|
|
|
What are the five main stages in the purification of 1-bromobutane after it's production from butan-1-ol?
|
Halogens are immiscible in water:
1)separate the organic layer and aqueous layer using a separating funnel 2)shake the product with sodium hydrocarbonate to remove any acid impurities 3)separate the product from the immiscible aqueous layer using a separating funnel 4)dry the product with anhydrous sodium sulfate 5)purify by simple distillation |
|
|
Advantages of batch process's
|
-Cost effective for small quantities
-Low capital cost for plants -Different products made using the same vessel |
|
|
Disadvantages of batch process's
|
-Filling cleaning and emptying reaction vessel is time consuming
-may require larger workforce -contamination possible if making different products |
|
|
Advantages of continuous process's
|
-Suited to high tonnage products
-Can operate for months at a time without a shut down -More easily automated,so workforce can be smaller |
|
|
Disadvantages of continuous process's
|
-Less flexible- usually designed. to make one product
-Much higher capital cost in building the plant -Not cost effective if run below full capacity |
|
|
What is the equation to work out percentage yield?
|
% yield = actual mass of product÷ relative formula mass of the product used × 100
|
|
|
What is the equation to work out atom economy?
|
% atom economy = relative formula mass of useful product ÷ relative formula mass of reactants used × 100
|
|
|
What are the differences between a by-product and a co-product?
|
-By-products are made through an unwanted side reaction
-Co-products are made through the desired chemical reaction, but it isn't the target product |
|
|
Suggests why locating a new chemical plant on an existing site of a chemical manufacture can lower costs
|
-The infrastructural such as a road network is already in place
-A skilled workforce is available |
|
|
Why might a company manufacturing bleach want to site it's self near a chlor-alkali plant?
|
Two of the products in the plant (sodium hydroxide and chlorine) are feedstocks in the production of bleach
|
|
|
What is a feedstock?
|
The reactant that go into a production process
|

