![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
20 Cards in this Set
- Front
- Back
|
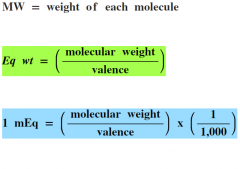
Define molecular weight (MW)
Define equivalent weight (Eq Wt)
Define milliequivalent weight (mEq) |
|
|
|
What is the age range for neonate? |
BIRTH – 1st MONTH |
|
|
UNITS ﹠ CONVERSIONS:
1 dL = _____ mL
|
1 dL = 1 deciliter
1 dL = 0.1 L
1 dL = (¹/₁₀) of Liter
1 dL = 100 mL
|
|
|
UNITS ﹠ CONVERSIONS:
1 DL = _____ mL |
1 DL = 1 Decaliter (DL)
1 DL = 10 L
1 DL = 10,000 mL |
|
|
UNITS ﹠ CONVERSIONS:
milli = (?) micro = (?) nano = (?) pico = (?) femto = (?) atto = (?) |
milli = 10⁻³ |
|
|
UNITS ﹠ CONVERSIONS:
|
1 g / 1,000 mL
(or)
1 mg /mL |
|
|
UNITS ﹠ CONVERSIONS:
1,000 mL of NS = _____ mEq |
154 mEq
|
|
|
What is the FREEZING POINT DEPRESSION of an isotonic solution? |
(#12–N)
🅰: –0.52 ⁰C
❶ An ISOTONIC solution depresses the Frezing Point to – 0.52 ⁰C
❷ NaCl = 0.9% = Isotonic = – 0.52 ⁰C |
|
|
BASIC CONVERSION:
milli, micro, nano, pico,femto, & atto are PREFIXES associated with which MEASURING SYSTEM? |
(#2-A)
Metric System International
(System International measuring system) |
|
|
MW﹠Eq Wt﹠mEq:
What are the VALENCE electrons for: ⁽¹⁾˸ Organic Acids ⁽²⁾˸ Protein |
(#9–A)
⁽¹⁾˸ Organic Acids = (-1) ⁽²⁾˸ Protein = (-1) |
|
|
PERCENTAGE (%) PREPARATIONS:
Asking for a (%) is the same as saying what? |
PARTS per HUNDRED
|
|
|
ABBREVIATION:
aa = _____ |
of each |
|
|
If a solution of a total of 4 LITERS requires 31 mL of Benzalkonium Chloride concentrate (17%), what would be your final solution in mL? |
(#5–A)
Since it is CONCENTRATED, you would NOT add it to the 4 L but instead 3,969 mL to make it a final solution of 4,000 mL.
(4,000 mL) – (31 mL) = 3,969 mL
|
|
|
DEFINE:
molarity ⨷ molality |
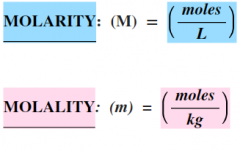
|
|
|
PERCENTAGE ﹠ RATIO STRENGTH: |
🅰: 11.1 %(w/w)
DISSOLVED = (1 gm) / (1 gm + 8 gm)
(#5–J) |
|
|
PERCENTAGE﹠RATIO STRENGTH: Normal Saline (NS) = _____ % (w/v) NaCl |
0.9% (w/v) |
|
|
ALLEGATION: |
(#6–F)
🅰: 4:5
(20) / (25) = 4/5 |
|
|
ALLEGATION:
❶ What is the chemical name for H₂O₂?
❷ At what PERCENTAGE STRENGTH of H₂O₂ does Normal Saline (0.9%) has? |
H₂O₂ = Hydrogen Peroxide
(0.9%) NaCl = "0%" of H₂O₂ |
|
|
ALLEGATION ﹠ PERCENTAGE STRENGTH:
❶: Water = _____ (%) v/v ❷: Vanishing Cream Base = _____ (%) w/v ❸: White Petrolatum = _____ (%) w/w |
❶: Water = 0% (v/v) ❷: Vanishing Cream Base = 0% (w/v) ❸: White Petrolatum = 0% (w/w) |
|
|
[⓫]: TPN﹠EN Calories
PROTEIN = _____ kcal/gm FAT = _____ kcal/gm CARBOHYDRATES = _____ kcal/gm DEXTROSE = _____ kcal/gm ALCOHOL = _____ kcal/gm |
PROTEIN = 4 kcal/gm FAT = 9 kcal/gm
CARBOHYDRATES = 4 kcal/gm DEXTROSE = 3.4 kcal/gm
ALCOHOL = 7 kcal/gm |

