![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
57 Cards in this Set
- Front
- Back
|
Additional Risk
|
Additional proportion of population that respond given conditional exposure
|
|
|
ADI
|
Allowable Daily Intake. Concentration of certain substance that can be taken, generally for a lifetime, and not show any adverse effects.
|
|
|
adverse
|
contrary to ones interests or welfare
|
|
|
Adverse Effect
|
Biochemical change or impairment that may affect well being of organism and its fitness
|
|
|
allometry
|
The study of natural human anatomical and physiological variation
|
|
|
Benchmark Dose
|
An exposure level that corresponds to a statistical lower confidence limit on the dose producing a predetermined response.
|
|
|
Bright Line:
|
specific value or line drawn as a boundary to separate acceptable vs unacceptable risk
|
|
|
Central Tendency
|
Descriptive term about accessing risk or groups that refers to mean, median.
|
|
|
Cancer Potency Factor
|
See Risk Factor
|
|
|
Critical Effect:
|
First adverse effect at the lowest exposure level; first adverse effect as dose increases.
|
|
|
Effect
|
response to a stimulus, outcome.
|
|
|
Excess Risk/Extra Risk
|
total proportion of additional risk responses caused by exposure that would not have that predetermined response.
total additional proportion of response compared to background proportion caused by exposure. |
|
|
Extrapolation
|
Through regression or other means, estimating a data point outside of our range of experimental values.
|
|
|
Hazard Quotient
|
The ratio of the dose resulting from exposure to an agent and the chemical’s reference dose (RfD) or other screening benchmark.
|
|
|
Health
|
State of an organism with respect to functioning, disease, and abnormality at any given time.
|
|
|
Health Effect:
|
Functional or structural response affecting the organism, but not necessarily leading to impairment or reduced capacity
|
|
|
Interpolation
|
Estimation of responses within the range of experimental data; determination of intermediate values in a series on the basis of observed values.
|
|
|
LOAEL
|
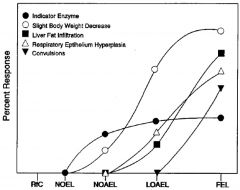
lowest observed adverse effect level. Lowest experimentally determined dose where a biologically or statistically significant adverse effect (critical effect) occurs compared to control
|
|
|
LOEL
|
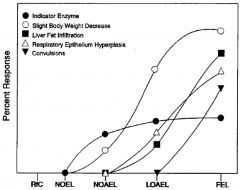
lowest observed adverse effect level. Lowest experimentally determined dose where a biologically or statistically significant effect occurs compared to control
|
|
|
MOE
|
Margin of Exposure. Ratio of NOAEL to the current estimated exposure dose of interest.
|
|
|
MOS
|
See Margin of Exposure
|
|
|
Mechanism of Action
|
Specific details of the biological processes (e.g., at the
molecular level) by which an agent causes its effects. |
|
|
Mode of Action:
|
Series of key events involving interaction with cells, leading to operational or anatomic changes, resulting in disease; an incomplete aspect of “Mechanism of Action”.
|
|
|
NOAEL
|
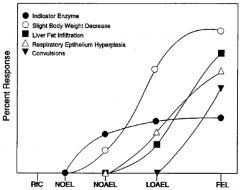
“No Observed Adverse Effect Level”: An experimentally determined dose at which no statistically or biologically significant indications of toxic effects (critical effect) are observed beyond those seen in a control group.
|
|
|
NOEL
|
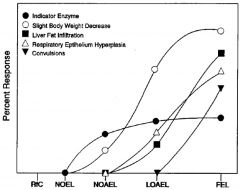
“No Observed Effect Level”: Highest experimentally determined dose at which no statistically or biologically significant effects are observed beyond those seen in a control group.
|
|
|
Pharmacodynamics
|
The study through measurement or modeling of the response of a biological system as a function of dose. Referred to as: “The linkage between dose and response” (NRC '94).
|
|
|
Pharmacokinetics
|
The study through measurement or modeling of the absorption, distribution, metabolism, and excretion of drugs or chemicals in a biological system as a function of time. Referred to as: “The linkage between exposure and dose” (NRC '94).
|
|
|
Point of Departure
|
The dose-response point that marks the beginning of a low-dose extrapolation. This point can be the lower bound on dose for an estimated incidence or a change in response level from a dose-response model (BMD), or a NOAEL or LOAEL for an observed incidence, or change in level of response.
|
|
|
Potency Factor:
|
Risk Factor
|
|
|
Q1*:
|
The upper bound on the cancer potency factor for low-dose exposures as calculated using the linearized multistage (LMS) model.
|
|
|
Residual Risk
|
That risk which remains after risk reduction actions have been implemented.
|
|
|
RfC
|
“Reference Concentration”: An estimate (with uncertainty spanning perhaps an order of magnitude) of a continuous inhalation exposure to the human population (including sensitive subgroups) that is likely to be without appreciable risk of adverse effects during a lifetime.
|
|
|
RfD
|
“Reference Dose”: An estimate (with uncertainty spanning perhaps an order of magnitude) of a daily exposure to the human population (including sensitive subgroups) that is likely to be without appreciable risk of adverse effects during a lifetime.
|
|
|
Risk
|
Probability of a specific, generally adverse outcome (e.g., injury, disease or death) given a particular set of conditions (e.g., exposure dose and route of exposure) and relative to the background probability.
|
|
|
Risk Assessment
|
Scientific activity of evaluating the toxic properties of a chemical and the conditions of human exposure to it in order to ascertain the likelihood that exposed humans will be adversely affected and to characterize the nature of the effects they may experience.
|
|
|
Slope Factor
|
Excess risk per unit of dose at a specific dose. Also referred to as the “Slope Factor” or “Potency Factor”.
|
|
|
Susceptibility
|
How much additional risk of an adverse outcome that can be attributed to some variable or exposure
|
|
|
Threshold
|
The line that separates adverse health effects and below no adverse outcomes are likely to occur.
|
|
|
Ultimate Toxicant
|
the final form of a toxicant that interacts on a cellular level to cause toxicity to life.
|
|
|
Unit Risk
|
The upper bound measuring excess risk at a standard unit point of (generally continuous) exposure.
|
|
|
Upper Bound
|
Statistical upper confidence interval limit, used for relevant risk measurements.
|
|
|
Threshold vs Non-Threshold Domains of Risk Analysis.
|
Risk analysis evolved into two domains:
cancer risk assessment = non-threshold non-cancer risk assessment = threshold i.e. for cancer, any amount is dangerous and carcinogenic |
|
|
Threshold toxicant
|
Substance known or assumed to have a threshold, below which no adverse health effects are expected; some exposure level is “safe“
|
|
|
NOAEL/LOAEL Model
|
One threshold Risk Assessment Model (two others)
FDA: Allowable Daily Intake (ADI) The amount of chemical to which a person can be exposed on a daily basis over an extended period of time (usually a lifetime) without suffering an adverse effect ADI = NOAEL / [Safety Factor] |
|
|
Tolerance Distribution Model
|
One threshold Risk Assessment Model (two others)
Statistical models that characterize the population dose response variability and estimate population response thresholds log-probit (probit) log-logistic (logit) Weibull (time to failure) |
|
|
Benchmark Dose Model
|
One threshold Risk Assessment Model (two others)
|
|
|
Inter-species Safety Factor (UFA)
|
Inconsistency of mechanisms across species
Addresses uncertainty of extrapolation from animals to humans, when human data are lacking Default = 10 |
|
|
Intra-species Safety Factor (UFH)
|
Variable responses between humans
Addresses uncertainty of extrapolation from general population to susceptible sub-groups Default = 10 |
|
|
LOAEL to NOAEL Adjustment Factor (UFL)
|
Inappropriateness of experimental end-point
Addresses uncertainty from using a LOAEL when NOAEL data are lacking Default = 10 |
|
|
Exposure Duration Adjustment Factor (UFD)
|
Inappropriateness of exposure duration
Addresses uncertainty from using sub-chronic data when chronic exposure data are lacking Default = 10 |
|
|
Sources of Uncertainty
|
Insufficient experimental data
Inconsistent experimental data Limited distribution or range of data Humans differ from experimental animals: Differing pharmacokinetics, Differing pharmacodynamics Humans differ among themselves Differing susceptibility Heterogeneity (genetic, lifestyle, etc.) Parameter uncertainty and variability:measurement error, random error, systematic error Model uncertainty: surrogate and excluded variables, incorrect model Decision-rule uncertainty |
|
|
NOAEL/LOAEL (safety factor) Model Advantages and Disadvantages (name at least three)
|
Advantages: Relative simplicity and Ease of application
Disadvantages: -Underutilization of available data. NOAEL is limited to tested data. Only one data point is considered. -Shape of dose response curve is ignored. -Size of study population is (usually) ignored -Reference Dose (rfD): indicates who is at risk not the size of the risk. -RfD biased statistic. UF conservative so RfD biased down. -Difficulties in interspecies scaling. |
|
|
EDn and ECn
LDn and LCn |
Critical effects for tolerance distribution model.
EDn and ECn are for dose and cxn causing a specific effect in (n% of pop) LDn and LCn are for dose and cxn causing a LETHAL effect in (n% of pop) |
|
|
Tolerance Distribution Model Steps
|
Find appropriate empirical dose-response data
Select a mathematical function that describes the empirical dose-response data. Calculate the goodness-of-fit Set a critical effect threshold |
|
|
Weight of Evidence
|
“Collective evaluation of all pertinent information so that the full impact of biological plausibility and coherence are adequately considered”
Considerations include: quality and quantity of data ability to detect effects (sensitivity), consistency of results across studies, biological plausibility |
|
|
Carcinogenicity Cancer Risk Assessment
|
Group 1: Definitely so
Group 2: Maybe so. 3: not classifiable, inadequate proof 4: Agent no carcinogenic. |
|
|
weight of evidence
|
Weight of Evidence
“Collective evaluation of all pertinent information so that the full impact of biological plausibility and coherence are adequately considered” EPA and IARC have different forms of measuring |

