![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
30 Cards in this Set
- Front
- Back
|
Lung volumes and capacities. |
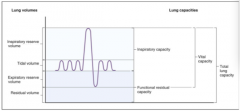
Adding up certain divisions of lung volumes creates varying groups of lung capacities.
|
|
|
Lung Volumes |
Tidal Volume (VT) - amount of air breathed in and out during one normal breath. ~15mL/kg for most domestic species.
Residual Volume (RV) - the volume of air that remains in the lungs after a complete and forceful expiration. You can't breathe it out no matter how hard you try to. |
|
|
Reserve Volumes |
Inspiratory Reserve Volume - amount of air that you can inspire beyond what you breathe in during a normal breath
Expiratory Reserve Volume - amount of air you can expire beyond what you breathe out during a normal breath |
|
|
Lung Capacities |
Vital Capacity - Maximum amount of air that can be inspired after the maximum amount of air has been expired (the most you can breathe in and out with max effort)
Total Lung Capacity = vital capacity + residual volume
Functional Residual Capacity = Residual volume + Expiratory Reserve Volume. The amount of air in the lung after a normal expiration. The lung volume that occurs when the chest wall and lung are in equilibrium. Affected by many diseases and conditions (diaphragmatic hernia, pneumothorax, etc) |
|
|
Why is energy used during breathing? |
1. Contraction of muscles expands the thorax and ventilates the lungs
2. Needs to overcome forces that would contract the lungs (recoil from elastic tissues, surface tension)
3. Needs to overcome resistance to air flow through the respiratory system. Everytime the airway forks, a little air turbulence is created |
|
|
Compliance |
Change in volume relating to a change in pressure. Measures how easy it is to distend the lung. Determined by measuring the change in lung volume as a result of a change in pressure.
|
|
|
Where is compliance highest? |
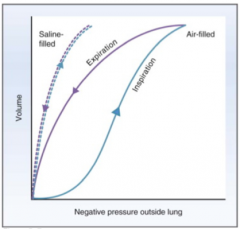
In the mid-range of pressure, compliance is high. Change in volume is large with a small pressure change.
At higher pressures (at the end of inspiration), compliance is low and the curve flattens out. Lungs are less distensible. |
|
|
Surface Tension |
Attractive forces between atoms or molecules. Water molecules pull on each other. Surface tension needs to be overcome in the lung for breathing |
|
|
Which law does alveolar surface tension follow? |
Laplace's Law P=2T/r P=the pressure inside the alveolus T=the tension on its inner surface r=the internal radius of the alveolus
P works to expand the alveolus T works to contract the alveolus |
|
|
Why is Laplace's Law important? |
The smaller the radius of the alveolus, the more pressure you need to overcome that initial resistance/inner surface tension (just like starting to blow up a tight balloon. Once you get it started it's a lot easier).
It also means that at a given surface tension, small alveoli would have higher internal pressures. As a result, they would always empty into the larger alveoli. This is not a good thing because we would expend a lot of energy just to breathe and air wouldn't necessarily go where it needs to. |
|
|
How do we reduce the work of breathing? |
Surfactant: a substance that accumulates at the surface of the fluid in the alveoli to reduce surface tension, thereby reducing the work of breathing. It reduces the # of water molecules that can attract each other. |
|
|
Pulmonary Surfactant |
Lipoprotein Complex: 70% lipid, 30% protein.
Synthesized by type ll alveolar epithelial cells. Surfactant producing cells develop very late in gestation, so premature babies don't have these cells and their lungs can't function properly. Another surfactant-lacking syndrome is Respiratory Distress Syndrome. |
|
|
At the end of expiration... |
The alveolus is at its smallest size (r is low). Surfactant becomes more concentrated on the surface of the alveolus, greatly reducing surface tension (T). Reducing surface tension also stabilizes the alveolus so that when it is small, it doesn't simply empty into larger alveoli and collapse. |
|
|
Surfactant Concentration vs. Alveolar size |
When the alveolus is small, like at the end of expiration, the surfactant is very concentrated so the surface tension is reduced (T). This counteracts the effects of the small radius so the 'opening' pressure at the start of inspiration is not prohibitively high, making it easier to start taking a breath. As the lung inflates with inspiration, that same volume of surfactant needs to spread over the larger alveolar area so the concentration of surfactant goes down. |
|
|
At the end of Inspiration... |
The alveolus is at its largest size (r is high). Surfactant spreads out (less concentrated) on the surface of the alveolus (surface tension, T, increases). This increased tension, along with elastin and collagen fibres, put an upper limit on the potential expansion of the lungs and provides some of the recoil necessary for making expiration a passive process. |
|
|
Airway Resistance |

Air flow follows the general principles of fluid flow through a rigid, smooth tube. Flow of fluid follows "Poiseuille's Law" n=viscosity of the fluid or gas l=length of the tube r=radius of the tube Small changes in diameter have a huge change on resistance, like breathing through a straw
|
|
|
Why is airway resistance important to consider? |
If the length of a tube is doubled, resistance will double and you need to double the force you apply in order to maintain a constant air flow.
However, if the radius is halved, the force must be increased 16X in order to maintain a constant flow.
Airway diameter has the greatest effect on resistance to air flow |
|
|
Where in the airway is resistance highest? |
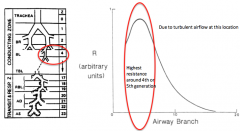
Greatest resistance is around the 4th-5th branches (lots of turbulence as airways divide). |
|
|
As you move down the respiratory tree, the branches get smaller and smaller. Why does airway resistance go down if the radius of the airways gets progressively smaller? |
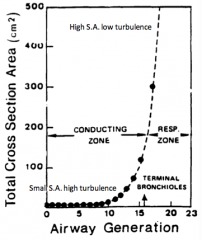
There are many more tubes as you get smaller and smaller, so there is a higher overall surface area. Higher surface area means less airway resistance as the surface area for gas exchange has increased. |
|
|
How does airflow resistance between the 2 zones compare? |
Resistance to airflow is lower in the respiratory zone than in the conducting zone, making it easier to breathe. |
|
|
Which gases are important for breathing? |
Oxygen Carbon Dioxide Nitrogen Water Vapour |
|
|
Gas Laws |
Volume = constant/Pressure volume of gas is inversely proportionate to pressure. As you dive deeper, volume decreases and pressure increases. Density also increases, making it harder to breathe
Volume = constant x Temperature Volume is proportional to temperature. Heat up air tank, pressure goes up
Dissolved amount of a gas = pressure x solubility coefficient Gas must dissolve in order to enter the bloodstream |
|
|
Partial Pressure |
The pressure exerted by a specific gas when it is in a mixture of gases (its contribution to the total pressure).
Identified by the symbol "P"
Calgary = 670 mmHG. 21% is oxygen |
|
|
Dalton's Law |
the pressure exerted by one gas is independent of the pressures exerted by the other gases in a mixture.
The sums of all the individual gas pressures in a mixture is equal to the "total gas pressure" |
|
|
How do you find the partial pressure of a gas? |
the P of a gas is found by multiplying its fraction concentration (%) by the total gas pressure:
Oxygen is 21% of the air we breathe. Total atmospheric pressure is 760mmHg. So the partial pressure of oxygen is PO2 = 0.21 x 760mmHg = ~160 mmHg |
|
|
How do we name the body's partial pressures? |
Pa = arterial pressure of a gas (amount that is dissolved in the plasma). The concentration of CO2 in the arteries is the same as in the lungs. It doesn't change because capillaries are required for a concentration change. Pv = venous pressure of a gas (amount dissolved in the plasma). The pulmonary artery is the best place to measure overall body venous pressure. Usually the jugular is used though because of easier access, but the reading won't be quite correct. PA = alveolar pressure (the air in the lung). The driving force to push oxygen across the membrane or to bring CO2 back across the membrane. |
|
|
Gas Movement |
Gases move by simple diffusion from areas of high pressure to areas of low pressure.
Regardless of the total pressure of a gas mixture, individual gases diffuse in response to their own gradients (from areas of high partial pressure to low partial pressure). Each gas follows its own gradient rules. |
|
|
Fick's Law |

|
|
|
What conditions favour optimal gas exchange in mammals? |
Large surface area
Fast diffusion coefficient
Large difference between the 2 partial pressures
Thin membrane thickness (single layer of simple squamous epithelium without significant cytoplasm or basement membrane) |
|
|
What can affect single layer membrane O2 and CO2 diffusion? |
pneumonia or pulmonary edema can thicken the layer by forming fluid overtop of it. This can affect diffusion and limit O2 and CO2 exchange. |

