![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
10 Cards in this Set
- Front
- Back
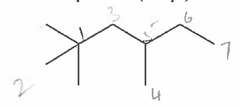
How many signals appear in the proton-decoupled 13C NMR spectrum of the following compound? |
7 |
|
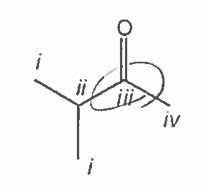
Which of the carbon atoms in the following molecule appears furthest downfield in the 13C NMR spectrum? |

iii |
|
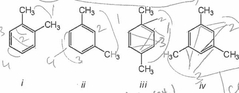
Which of the following compounds would give a 1H NMR spectrum consisting of two singlets and a 13C NMR, consisting of three signals? |
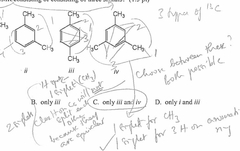
Only iii and iv. |
|
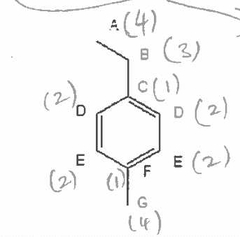
Predict the splitting pattern in the proton coupled 13C NMR spectrum for the following compound. |
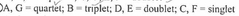
|
|

Which of the following is the initiation step for the monobromination of cyclohexane? |
IV |
|
How many products are formed from the monochlorination of ethylcyclohexane? Ignore stereoisomers. |
6 |
|

Given the bond dissociation energies below (in kcal/mol), estimate the ΔH° for the propagation step. |
-22 kcal/mol |
|
|
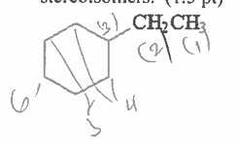
Which of the following is a chain propagation step in the free radical chlorination of methane? Which of the following is a chain propagation step in the free radical chlorination of methane? |
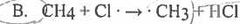
|
|

|
V |
|
|
In the free radical chlorination of ethane, the step in which the CI radical abstracts a H atom from ethane is ______ and the transition state most closely resembles ________. |
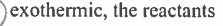
|

